Root Methods
Introduction to Common Root Phenotyping Methods
Root observation and phenotyping is often an important and a required part of abiotic stress research, especially in breeding and selection. The available information on methods of root research is very large, scattered and often difficult to resolve towards one’s work. Here, several of the more common and significant methods are described as an entry point.
The breeder will recognize that most methods (especially in the field) are not fast enough for routine selection in large populations. Most methods are at least appropriate for pre-breeding work while others can be used to screen large populations. However, when drought resistance is considered, root function can be phenotyped indirectly by assessing plant water status in a drought managed breeding nursery (see Blum, 2011) rather than by accessing the root itself.
FIELD METHODS
Trenching
This method is not very popular for obvious reasons. It might be practiced in certain cases especially when in situ root observations are required with respect to certain soil or root problems – suspected or real. Sometimes after trenching a pinboard is pressed against the trench wall and with, the appropriate equipment a “slice” of soil, which contains soil and root as, held by the pins is lifted and brought to the lab. Soil is then washed off and the remaining roots, which are held by the pin board, are observed, measured and photographed. Recently, spot observations in the field are done by use of industrial tractor mounted excavators.
Soil cores
 |
Soil coring hand tools (from Soil Moisture Equipment Co.) |
When deep distribution of roots in the soil is of interest soil cores are extracted from the desired depth. Various hand tools are available to suite the required job and soil characteristics. For detailed suppliers see our “Web Resources” page and then check “Materials and Services” lists. Besides hand tools, heavy work especially in heavy and hard soils has been performed by hydraulically operated systems. These are usually tractor operated and are not suitable for work within a small experimental plot which has to remain intact. Cores are extracted from a set of points in a plot or around the plant or around the row as the case might be and according to the expected spread of the root system. Cores are usually extracted in sequence in depth interval of 15 to 30 cm.
When deep distribution of roots in the soil is of interest soil cores are extracted from the desired depth. Various hand tools are available to suite the required job and soil characteristics. For detailed suppliers see our “Web Resources” page and then check “Materials and Services” lists. Besides hand tools, heavy work especially in heavy and hard soils has been performed by hydraulically operated systems. These are usually tractor operated and are not suitable for work within a small experimental plot that has to remain intact. Cores are extracted from a set of points in a plot or around the plant or around the row as the case might be and according to the expected spread of the root system. Cores are usually extracted in sequence in depth interval of 15 to 30 cm.Whatever core extraction method is used, cores have to be washed in order to extract the maximum amount of root from the sample – usually for the measurement of total root length in the sample. When total root length per core is measured against core volume, it is possible to estimate root-length density (cm cm-3) for that core. Data from all cores can provide a reasonable estimate of total root length of the plant or for the given soil horizon, depending on the case. Cores can also provide a good estimate of root depth and root distribution along soil depth.
Root washing of soil cores can be done by dipping and washing each sample in water. In heavy soil, it might be necessary to soak samples overnight in a saturated NaCl or soap solution to increase buoyancy and disperse soil aggregates. Distinguishing between live and dead roots (or residues) is usually done subjectively based on criteria such as color. Mechanical systems for root washing are available commercially in limited variety and the hydropneumatic elutriation system by Gillisons seems to be in more common use. It is based on an early design by Dr. Smucker from Michigan State University in the early 1980’s. A modified system has been designed later by Chotte et al.1995.
The washed root samples can be measured for total root length and other morphological features using standard imaging and image processing systems. A simple outline of the work is described by an online publication from the PSU root research lab. A popular method for root image acquisition and analysis is , a product of Regent Instruments. It is briefly described in that PSU publication.
Shovelomics
By simply using a shovel combined with imaging technology this method was effective in phenotyping the top part of a crown root system of mature maize. For that purpose, the method was defined as high throughput field method. For each sampled plant, the root was excavated by extracting a soil cylinder of about 40cm in diameter and 25 cm deep with the plant crown in the middle. This off course will not work in a very dry and hard soil. The soil was shaken off the roots after which it was dipped in water and a detergent to remove the remaining soil. The exposed root was measured for various trait of significance to this study and crop, such as number of crown root wholes above and below ground, crown root number etc.’ The extracted roots can also be stored under refrigeration and phenotyped over time. It is also easy to imagine that for certain purposes, it might be sufficient to visually score certain root morphological traits. Fig.2 in the paper represents well the very large variation for different root traits among 218 recombinant inbred lines of maize that could be captured by this simple method. While the method recovered only the top part of the root system these photographs demonstrate how additional root traits could have been interpolated for the different phenotypes.
Mini-rhizotron
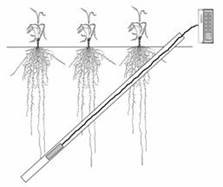 |
Minirhizotron schematics |
The method has gained popularity as a leading qualitative and quantitative field observation system. It is based on a transparent glass or plastic sleeve inserted at an angle (usually 450 to the vertical) into the soil before roots become very developed. The sleeve is inserted into a borehole made manually or with hydraulic assistance. The procedure is important since good contact must be achieved between the sleeve surface and the soil. An imaging device, whether a digital camera, a video camera or an endoscope is inserted into the sleeve in order to record images of roots seen through the sleeve walls. The images are then recorded and processed by an aboveground unit. Depending on the system and model, images can be processed within this unit or downloaded to a computer equipped with the appropriate image processing software. In many cases, images are processed for root length, thickness and other parameters by the WinRhizo software as one example. Free and open-source software for analyzing minirhizotron images is available also, such as the from Clemson University. It is off course understood the protocol for acquiring the images is linked to the specific image analysis software used. Root images seen on the sleeve wall are not a perfect representation of the actual root distribution at that soil horizon and the software attempts to simulate real root data from the images. Often, several soil cores are also taken (see above) for verification and correlation with minirhizotron observations. Several studies have indicated that the correlation between minirhitotron data and soil coring data is very poor at the top soil, for a variety of reasons. Researchers tend to disregard top soil minirhizotron data.
Barz Technology Corp. is a recognized supplier of various minirhizotron models as well as additional important ancillary equipment. Commercial systems are convenient, reliable but expensive. Simpler self-made units are known to have been used, as seen in the website. Digital cameras and image capture systems are becoming less expensive and more dependable, so that “home-made” units can be quite effective, depending the specific job. It goes without saying that for using a minirhizotron for root observation and data collection one must obtain good background information, and sometimes even training. Beginners are encouraged to read the appropriate chapters in Root Methods by Smit et al. 2000.
Root pulling force for estimating plant anchorage
 |
A simple installation for measuring root-pulling force of rice in paddy. (Photograph courtesy of Dr. John O’Toole, IRRI). |
The measurement of in situ plant resistance to vertical pull was initially and mainly developed as an indirect assessment of various maize materials for lodging resistance. Maize adventitious root number, depth and strength determine the “root lodging” trait where plants lodge due to poor root anchorage in the soil. Various simple methods were used for measuring maize root pulling force. The stalk was cut some 40-60 cm above ground. A clamping device was connected to the stump and then to a tensiometer or an electronic strain-gage which was pulled by a manual or a hydraulic lever. Force required to pull the roots out of the ground was recorded and was found to be well correlated with lodging resistance. Root pulling force was also related to rootworm resistance under soil-infested conditions.
Root pulling force was first used in rice by O’Toole and Soemartono (1981) as an indirect estimate of root related dehydration avoidance capacity. Ekanayake et al (1985) found a significant positive correlation across diverse rice genetic materials between root pulling force and dehydration avoidance as expressed in leaf water status maintenance and visual scored of drought resistance under severe drought stress in the field. Long fibrous roots have long been recognized as an important dehydration avoidance mechanism in rice and such roots evidently also ascribe stronger anchorage and greater resistance to pulling force. The method appears sensible for root depth phenotyping in rice, especially when other direct field methods for root selection are not very forthcoming. Occasional additional studies have been reported on using root-pulling force as an estimate of drought resistance in rice and maize but the method did not gain wide popularity in selection work for drought resistance.
OUT-OF-FIELD METHODS
Observation and measurement of roots in various containers
Root growth and development in a container is rarely perfectly representative of root growth in soil in the field – especially where special soil conditions may exist. Container studies of roots are therefore a compromise, which in certain circumstances can be very effective for the specific purpose of the study.
The prime consideration in container-grown roots is to minimize root growth restriction by the container. While roots in containers will always touch the walls, insufficient depth of the container is a major reason for root growth bias from the normal. The second consideration is the root growth medium that has to be reasonably dense so as to offer the natural resistance offered by the average normal soil in the field. Roots growing under small resistance can grow to abnormal size. The extreme case is a root grown in aeroponics which can reach an unnatural huge size. On the other hand, very heavy clay soil which tends the shrink when dehydrated is inappropriate when soil drying is an experimental treatment. When roots are to be extracted from the containers and washed for measuring root traits the medium has to be perfectly clean of debris and amenable to easy wash. Most of the other considerations such as absolute medium bulk density, fertilization, soil moisture control etc’ can be considered as experimental variables.
Boxes and panels
 |
A glass-paneled in-ground root box/lysimeter installation allowing measurement of roots and transpiration. (Texas A&M Research Center at Temple, TX). |
Growing plants in large boxes equipped with a transparent glass or polycarbonate wall has been used extensively in the past. The transparent panel might be vertical or slanted at an angle while the visible roots were recorded periodically as representative of the whole root system. Roots within the box could be extracted, washed and measured for various traits. In certain cases, boxes were weighed periodically to calculate plant water use. Pioneering work on wheat roots in relations to drought resistance and field performance was done in glass-paneled boxes by Hurd in Canada in the early 1970’s. Blum and Arkin (1984) upgraded the root boxes concept for sorghum by building large glass-paneled metal boxes, which were set in a sleeve inside the ground as part a full crop canopy in the field while boxes could be lifted for weighing, and root observations. This was actually a combination of the root observation box as it is common in full-fledged rhizotron installations with the field lysimeter. By washing and measuring the roots in several boxes, a correlation could be established between total root length measured periodically on the glass panel and real root-length density in the box. Periodical observations on the glass panel also allow accounting for and calculating root senescence according to root disappearance after a period of time. A typical representation of irrigated sorghum root development through maturity as recorded on the glass panel of one root box is presented here. Such a system can be upgraded by using image processing and software for faster and better root data calculation (see below).

The combined root observation unit with a weighing lysimeter has been evolved into a system of weighed tubes as seen at ICRISAT, India. Plants are grown in long tubes appropriate to root length. Tubes are placed in a trench in the ground so that can be lifted for weighing (as seen for the root boxes in the photograph). The whole installation can be placed under a rainout shelter so as to manage the drought stress conditions in the experiment.
Tubes etcetera
A very common and inexpensive container, which is widely adopted for root phenotyping in breeding work, is the PVC tube. This is a standard plumbing pipe cut to length and plugged on one end with the appropriate commercial fitting. Common diameter used is 4” but other sizes were reported depending on purpose and crop plant. The tube can be drilled for drainage and for inserting various sensors into the medium. The tube can be sawed longitudinally and then held by strong tape before filling it with the medium. At any point in time the tape can be removed and the tube opened for observations or root wash. Theoretically if roots are not damaged or disturbed (and the medium not dried up) the tube can be taped back again and setup for the continuation of growth. Roots or root samples extracted and washed from these tubes can be measured using the system and software as described above. For special purposes transparent tubes made of glass or polycarbonate were used. These are expensive and popular in special installations for high throughput phenotyping of roots where repeated observations (usually by remote sensing and imaging) are required. The color of the medium has to be compatible with the imaging system so that roots will be well defined in the output. A further modification of the transparent tube is by using transparent agar based nutritive medium so that plant roots can be seen and imaged within the tube and not only over the surface of the medium. Root development in the soft agar medium might be unnatural but it seems that at least for rice this system could capture basic potential genetic differences in the root phenotype. The original work was limited to plants growing in small 2 L glass cylinders.
Other systems quantify and characterize roots growing in soil in containers by taking X-ray images of the root in situ.
High throughput commercial plant phenotyping operations which are largely based on automated systems in greenhouse facilities combine plant growth in transparent tubes with shoot and root architectural imaging, plant water-use, plant water status and more – into a complete single plant phenotyping. The individual researcher can certainly adopt choice components of these systems in the execution of his/her own experiments. However, with the very advanced development of imaging and image analysis technology certain commercial systems might actually provide an expensive “overkill” of data on roots which might not be always necessary for the specific project and goal. Before pursuing expensive root data collection on your own or through commercial providers first ask yourself what is the root information that you really need.
On the other hand, breeders have been using commercial PVC sleeves used for the production of transparent PVC bags of various diameters. Sleeves were cut to length and filled with a growing medium. Seed were planted and thinned to one plant per sleeve. Sleeves were stood in a vertical are angled position with the aid of simple support system in the greenhouse. At any point in time, roots could be observed through the transparent PVC or the sleeves could be sliced open and roots washed out. This inexpensive system is sufficient for most practical breeding applications.
Hydroponics
 |
Hydroponics system with saline nutrient solution – one entry survived. |
Roots can be easily observed, sampled or measured non-destructively when the plant is grown in hydroponics. Root development may not be comparable to that grown in soil but for certain applications this system might be acceptable. A suitable container for root hydroponics is again the tube. In this case a sidearm with a fitted graduated pipette at the top can serve well to measure solution level (see photo). Aeration of the solution is necessary. In certain cases, root-compatible fungicides may be added to the solution in order to avoid bio-contamination. It is however recommended to replace the solution occasionally, depending on root size and solution volume.
The advantage of hydroponics is the accessibility to the root for measurements. While most forms of root measurements are possible, the main advantage is in being able to measure also root volume. This is a simple method based on classical water displacement. It has been shown that root volume was highly correlated with total root length as estimated by the WinRHIZO method.
Simulated drought stress can be imposed on hydroponics systems by two methods. First, the nutrient solution can be supplemented by PEG in order to reduce the osmotic potential of the medium. The method is acceptable pending many conditions and precausions. Secondly, the nutrient solution level can be allowed to be drawn down by transpiration or actively reduced in its level so that only the lower part of the root will be immersed in the solution. Under sufficient evaporative demand, such plants will not be able to take up enough water and stress symptoms will develop. With this method, care must be taken to maintain daily the desirable level, concentration and pH of the nutrient solution.
Other stresses such as salinity (see photo), mineral deficiency or toxicity are relatively simpler in application under hydroponics systems. There are however some controversies in the literature on the correlation between response to stress in hydroponics and in soil. The corollary in all cases is to apply stress slowly for a sufficient long time in order to allow for adaptation and acclimation.
A novel semi-hydroponics system was developed by Chen et al. (2011) for screening Lupinus angustifolius germplasm. With this simple and inexpensive system roots grow over a vertical black calico cloth wetted by the nutrient solution and therefore are exposed to imaging and measurements. Another argued advantage of the system is that it takes little space. More experience with this system might be acquired with time.
Root penetration capacity
 |
Phenotyping rice root penetration capacity using the wax layer system (Photo courtesy Dr. H.T. Nguyen) |
Soil impediments that offer resistance to root penetration of the soil constitute a serious limitation to root capture of soil moisture and nutrients. Hence, root penetration capacity can be considered as an important factor in avoiding drought and mineral nutrition stress where soil impediments exist. The subject is difficult. Without resolving to a long discussion of the literature it can be concluded, for now, that the superior capacity of a single root axis the overcome resistance to penetration is not absolutely clear.
It seems to be a consensus that there is a general positive correlation between root penetration capacity and root thickness (e.g. Materechera et al., 1992; Price et al., 2000) and/or vertical rather than angled root axis growth habit (e.g. Kato et al., 2006). Furthermore sloughing root cap cells and root exudates serve to lubricate root penetration path in the soil. An in-depth review of the biophysical aspects of root penetration is given by Bengough et al., 2005. An earlier review also provides an insight into the methods available for researching the response of a single root to penetration resistance.
On the whole plant level and for phenotyping work in breeding, the root penetration of a wax layer seems to gain popularity as a method of preference. The method is based on the number of roots in relations to total number of roots per plant able to penetrate a wax-petrolatum layer placed at a certain depth below the plant. The method was conceived by Taylor and Gardner in 1960 and was introduced into phenotyping work of rice by Yu et al. in 1995. The method was then applied to cotton, wheat, and possibly other crops.
In rice, as an example, the wax layers were prepared by mixing melted wax and petrolatum at a ratio of 3:2 by weight. Each layer had a penetrometer strength of 1.4 MPa at 27°C, weighed 50 g and was 13.0 cm in diameter and 5 mm thick. One plant was grown in each PVC pot (17.5 cm high and 16.0 and 12.0 cm in diameter at the top and bottom, respectively) with the bottom removed. A circular plastic crate (13.5 cm diameter with 1 cm2 perforations) was installed in the bottom of each pot. One wax-petrolatum layer was placed immediately above this crate. The gaps between the wax-petrolatum layer and the sides of the pot were sealed using melted wax. The pots were filled with fertilized potting mixture and then suspended in tanks filled with water up to 4 cm from the bottom of the pot (see ). Other specifications might be required with different rice populations or different crop species. Clark et al. (2008) noted that multiple wax layers which offered a gradual increase in resistance provided a better prediction of root penetration capacity in the soil, probably due to the expression of a hardening effect. While the wax penetration method is not always perfectly predictive of soil penetration it is still accepted as a reasonably relevant method. In the cereals it should be noted that differences exist in the basic penetration capacity between seminal and adventitious (nodal) roots. Application of the method in wheat and its relations to field performance can be found in Acuna, He and Wade, 2012 and references therein.
Root hairs
Root hair density and length are important traits in root capture of nutrients and water. using image analysis is well described in a detailed report from the Root lab at Penn State University.
Root hydraulic conductance
A reasonable entry point to understanding root hydraulic conductance measurement and the associated issues is the paper by Miyamoto et al., 2001. A co-author of this paper is E. Steudle, a well known authority on the subject. The paper describes the two main methods used in most root conductivity experiments, namely the pressurized whole root system and the pressure probe. The former uses whole plant with intact root system which was grown in hydroponics. The latter uses small root sections under the microscope. Both methods are not absolutely free of criticism, depending on their use, application and interpretation.
Root Porosity
Air-filled porosity of the root cortex is a crucial determinant of root growth under conditions of anoxia. Air-filled porosity is an important component of plant resistance to water-logging (e.g. Mitigation/Plantstress). Air-filled root tissue (“aerenchyma”) is characteristic of many plant species capable of coping with water-logged conditions. Van Noordwijk and Brower (1988) described and compared two methods for measuring the air-filled porosity of roots. Visser-and-Bogemann (2003) suggest a root porosity measurement technique with very small root samples.
Root analysis software
Commercial
Non-Commercial
Root Image Processing Lab (courtesy Michigan State Univ.)
Archiroots web site – mainly simulations
Few additional readings
- Weaver J.E. 1926. Root Development Of Field Crops.Mcgraw-Hill Co., New York; (if you are in a nostalgic mood).
- Smit et al., 2000. Root Methods, Springer.
- Mancuso Stefano (ed.), 2011. Measuring Roots: An Updated Approach; Springer.
- PSU Root Lab– Some detailed root methods online.
- Shashidhar HE, Henry A, Hardy B, (eds.) (2012). Methodologies for root drought studies in rice, International Rice Research Institute
- Marta Lopes, J. Israel Peraza Olivas and Manuel López Arce (2012). Sampling soil for moisture, nutrient and root content, from CIMMYT.
Disclaimer
Mention of trademarks and/or suppliers of equipment and materials do not constitute a recommendation or an endorsement by Plantstress.com.








