Mitigation of Cold Stress
Drs D. B. Fowler and A. E. Limin, Crop Development Centre University of Saskatchewan Saskatoon, Saskatchewan, S7N 5A8 Canada
Crop Plant Resistance to Freezing Stress
Genetic control of low-temperature tolerance
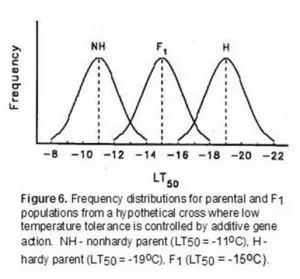
Low-temperature tolerance is a complex quantitative character that is expressed following exposure of plants to temperatures that approach freezing. While a large number of studies have been conducted, there is not a general consensus on the mode of gene action controlling the expression of this character. Recessive, additive, partial dominant and overdominant control have all been reported for genes conditioning low-temperature tolerance. The limited resolution of differences in low-temperature tolerance with field and controlled-freeze tests that employ a single minimum stress level have been at least partially responsible for some of the contradictory conclusions reported for genetic studies. For example, consider a hypothetical genetic study where the differences in low-temperature tolerance between two parents are controlled by additive gene action (Figure 6).
In this example, if a single minimum temperature of a controlled-freeze test or field trial was -13oC, most of the F1 and all of the hardy parent population would survive suggesting that there was dominance for low-temperature tolerance. Similarly, a single minimum temperature of -17oC would suggest that low-temperature tolerance was a recessive character. In this example, use of both -13oC and -17oC in a controlled-freeze test could lead to the mistaken conclusion that low-temperature tolerance was determined by dominant gene action under low levels of stress and recessive gene action under high levels of stress. A series of test temperatures that identifies the LT50 (temperature at which 50 percent of the population is killed in a controlled-freeze test) of each population reduces the likelihood of this type of error.
In spite of the limitations imposed by screening methods, the results of numerous research studies (Grafius 1981, Stushnoff et al. 1984, Blum 1988, Limin and Fowler 1991) with field crops can be summarized to provide a general picture of the genetics of low-temperature tolerance, the range of genetic variability for gene pools within species, the potential sources of new exploitable genetic variability, and the expression of superior low-temperature tolerance genes introduced into alien genetic backgrounds. The results of these studies demonstrate the complex and multigenic nature of the mechanism controlling low-temperature tolerance.
- a) Cytoplasmic factors have been implicated in the control of low-temperature tolerance. However, most studies have concluded that cytoplasmic differences are of minor importance and, if involved at all, play a secondary role in the low-temperature tolerance control mechanism.
- b) Genes conferring different levels of low-temperature tolerance are found within and among species. Plant breeders have been able to successfully manipulate this variability to maintain cold hardiness levels of cultivars within established production areas. Considerable variability in the range of low-temperature tolerance also exists among species; however, attempts at interspecific and intergeneric transfers have produced discouraging results.
- c) Although there are examples of nonadditive gene action, low-temperature tolerance within species is controlled mainly by genes with additive effects. In wheat, the identification of a dominant gene(s) affecting low-temperature tolerance that is tightly linked to vernalization (Vrn1) (Brule-Babel and Fowler 1988, Sutka and Snape 1989) and prostrate growth type (Roberts 1990) genes on chromosome 5A has proven to be an important exception to the additive gene action rule. Within the Triticeae, the genes for vernalization are found on the 4th, 5th, and 1st group chromosomes (McIntosh et at. 1998) while the 4th and 5th group chromosomes are most commonly associated with low-temperature tolerance (Sutka 1981, Law and Jenkins 1970). Subsequent work with barley has identified a linkage between low-temperature tolerance and vernalization genes on chromosome 7 (Hayes et al. 1993), which is homeologous with the 5th chromosome group in wheat. These linkages have provided valuable phenotypic markers for the investigation of low-temperature tolerance in Gramineae.
- d) Superimposed upon both quality and quantity of low-temperature tolerance genes is the effect of cell size. Smaller cell size amplifies the expression of low-temperature tolerance genes during the acclimation process (Limin and Fowler 1989). In winter wheat, although cold-acclimated cells are smaller, cell size rankings of cultivars follow a similar order for acclimated and nonacclimated plants indicating that differences in this character are intrinsic to the cultivars and not just low-temperature induced. Control of cell size has been localized to the Vrnregion of the group 5 chromosomes in wheat (Limin and Fowler 2001).
- e) A high degree of genetic balance or harmony is required for full expression of genes central to the low-temperature tolerance mechanism. The unpredictable low-temperature tolerance of artificially synthesized ABD genome hexaploid wheat (Limin and Fowler 1982) demonstrates the nonadditivity and asynchronous behaviour of related but unintegrated genetic systems. In synthetic amphiploids of the Triticeae tribe there appears to be a chromosome (gene) dosage effect that favors the expression levels of low-temperature tolerance genes from the parent species contributing the larger chromosome number (Limin and Fowler 1991). Observations made at this level have led to the suggestion that some degree of genomic integration, which would have been accomplished by recombination and (or) mutation followed by selection, was necessary before maximum low-temperature tolerance was achieved in naturally occurring polyploids of the Triticum– Aegiolops group (Limin and Fowler 1989). These complex interactions should not be unexpected, as there is evidence that certain regulatory mechanisms, such as species specific tRNA and mRNA promoters and interacting transcription factors, may have coevolved in eucaryotic genomes (Watson et al. 1987). However, the need for a highly integrated genetic system to maximize gene expression within species does not bode well for efforts to expand gene pools for low-temperature tolerance through interspecific and intergeneric cytogenetic introgressions or the production of transgenics using biotechnological techniques.
- f) Specific gene interactions, such as homoeoallelic dominance or threshold effects (Limin and Fowler 1991), may play a role in the final expression of low-temperature tolerance genes introduced into alien genetic backgrounds. As an example, the superior low-temperature tolerance of rye is suppressed when combined in tetraploid (Limin et al. 1985) and hexaploid (Dvorak and Fowler 1978) wheat backgrounds. These observations once again emphasize the difficulties that can be expected to be associated with efforts to provide breeding programs with superior low-temperature tolerance genes by interspecific and intergeneric transfers using cytogenetic or transformation procedures.
Developmental Regulation
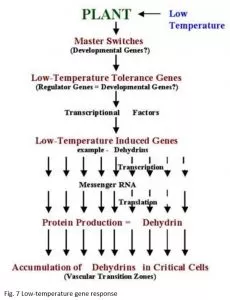
The genetic system that determines low-temperature tolerance can be divided into three separate components for discussion purposes. The master switches that are integrated into the mechanism that regulates plant development e.g., vernalization and photoperiod. Low-temperature tolerance genes that have been identified in conventional genetic and cytogenetic studies. Low-temperature induced genes that have been identified through differential screening of cold-acclimated wheat cDNA libraries (Figure 7).
The ability to survive cold winters and continue growth at near freezing temperatures provides a species with a competitive advantage by lengthening the effective growing season or positioning the plant to capitalize on favorable weather periods during the growing season. In order to cope with this stress, plants have evolved reversible acclimation systems that are light and temperature regulated. In areas with long, mild winters, a day length (photoperiod), dormancy, or low-temperature (vernalization) requirement that prevents plants from entering the extremely cold-sensitive reproductive growth stage until the risk of low-temperature damage has passed are the most important adaptive mechanisms. As a result, the evolution and selection of genetic options that permit extensive modification of temperature sensitive metabolic processes and critical structural components is of great concern in the successful adaptation of plants that must survive a wide range of seasonal challenges.
Low-temperature tolerance in cereals is dependent upon a highly integrated system of structural, regulatory, and developmental genes. In regions with cold winters, vernalization requirement is an important adaptive feature that delays heading by postponing the transition from the vegetative to the reproductive phase. Similarly, photoperiod requirement is an adaptation that allows the plant to flower at the optimum time. Time sequence studies have shown that low-temperature gene expression is also developmentally regulated (Fowler et al. 1996b). In this system, transition from the vegetative to the reproductive growth stage is a critical switch that initiates the down regulation of low-temperature induced genes (Fowler et al. 1996a, b, Mahfoozi et al. 2001). As a result, full expression of cold hardiness genes only occurs in the vegetative stage and
plants in the reproductive phase have a limited ability to cold acclimate. In addition, plants that are still in the vegetative stage have the ability to re-acclimate following periods of exposure to warm temperatures while plants in the reproductive phase have only a limited ability to re-acclimate.
According to the developmental theory (Fowler et al. 1999), level and duration of gene expression determine the degree of low-temperature tolerance. The developmental genes (vernalization, photoperiod) act as the master switch controlling the duration of expression of low-temperature induced structural genes (Fowler et al. 1996a, b, Mahfoozi et al. 1998) while the level of low-temperature tolerance is determined by the length of time and degree that the structural genes are up-regulated. Vernalization requirements allow low-temperature genes to be expressed for a longer period of time at temperatures in the acclimation range (Fowler et al. 1996a, b). Similarly, photoperiod sensitivity allows plants to maintain low-temperature genes in an up-regulated state for a longer period of time under short day compared to long day environments (Mahfoozi et al. 2000). In both instances, the delay in the transition from the vegetative to the reproductive stage produces increased low-temperature tolerance that is sustained for a longer period of time. This observation also explains why a high level of cold tolerance has not been observed in spring habit cultivars. Because low-temperature gene expression is only up-regulated when the plant is in the vegetative stage, the genetic potentials of spring habit cultivars are not given an opportunity to be fully expressed leaving the impression that the spring habit Vrn1 allele has a dominant pleiotropic effect for frost susceptibility.
Low-temperature induced genes
Winter cereals produce several proteins in response to low-temperature stress. Among these low-temperature induced proteins, the dehydrin families have received the most attention in recent low-temperature research (e.g. Wcor 410, Wcs120, dh5, and others). As a group, dehydrins have a wide size range, have no similarity with any enzymes or proteins of known function, are largely hydrophilic, and accumulate to high levels during the late stages of embryogenesis or in response to ABA application, low-temperature, or any environmentally imposed dehydrative force such as drought, intracellular freezing or salinity (Close 1996, 1997). For example, the WCOR410 family are peripheral acidic dehydrin proteins found near the plasma membrane and not integral membrane proteins. Immunoelectron microscope analyses of cold acclimated plants has revealed that proteins of this family accumulate in cells of the sensitive vascular transition area where freeze-induced dehydration is likely to be more severe (Danyluk et al. 1998). Their properties, abundance, and localization suggest that they are involved in the protection of critical membranes by replacing water and stabilizing membranes against freezing or dehydration stress.
The low-temperature induced dehydrin gene families have been studied extensively in wheat where they have been mapped to the group 6 chromosomes (Limin et al. 1997, Danyluk et al. 1998). Expression studies with the low-temperature induced Wcs120 and Wcor410 dehydrin gene families indicate that, even though there are large differences in low-temperature tolerance, similar proteins are expressed by spring and winter-habit cultivars within species (Houde et al. 1992, Fowler et al. 1996a, Danyluk et al. 1998). Cold hardy genotypes just produce more of the same dehydrins than tender genotypes. This indicates a common regulatory control in the level of expression of these structural genes in both hardy and non-hardy genotypes suggesting that the important regulatory factors affecting all low-temperature tolerance associated genes may not be as multigenic in nature as once thought. Further support for this conclusion can be drawn from the report that a single transcriptional factor has been found to regulate the expression of several low-temperature regulated genes in Arabidopsis (Thomashow 1999).
Low-temperature tolerance genes/Master switches
Because of the large number of chromosomes that have been shown to influence low-temperature tolerance in conventional, non-molecular genetic studies, it has been generally assumed that a large number of genes with small effects and complex interactions determine the phenotypic expression of low-temperature tolerance in cereals. However, even with the availability of molecular mapping tools it does not appear that cause and effect relationships will be easily established. To date, molecular mapping studies have only succeeded in locating one low-temperature tolerance gene (designated Fr1 by Sutka and Snape 1989) that appears to be tightly linked to the vernalization gene, vrn1, of chromosome 5A in wheat (Galiba et al. 1995). Earlier studies had identified a gene in this region with a dominant effect for low-temperature tolerance that was normally expressed in association with the recessive vrn1 allele for winter growth habit in wheat (Brule-Babel and Fowler 1988). Vrn1 is homoeoallelic to locus Sh2 in barley (Hayes et al. 1993) and Sp1 in rye (Brule-Babel and Fowler 1989), both of which have been linked to genetic differences in low-temperature tolerance.
The group-5 chromosomes carry the vernalization alleles vrn1, vrn4, and vrn3 on chromosomes 5A, 5B, and 5D, respectively. Substitution of each of these chromosomes from a hardy winter cultivar into a nonhardy spring cultivar reduced cell size without affecting growth habit (Limin and Fowler 2001). Genes on the 5th group chromosomes are also known to affect low-temperature tolerance. The Vrn1 region of chromosome 5A and homoeologous loci in wheat and other cereals appear to play an especially important role in determining plant responses to stress. Low-temperature tolerance and other stress tolerance related characters that have been associated with Vrn1 or homoeologous chromosomal regions include: antifreeze protein accumulation (Griffith et al. 1997), sucrose accumulation (Galiba et al. 1997), ABA accumulation (Galiba et al. 1993), unsaturated phospholipid synthesis (DeSilva 1978), prostrate growth habit (Roberts 1990), cell size (Limin and Fowler 2001), low-temperature tolerance associated with the Wcs120 and Wcor410 gene families (Limin et al. 1997; Danyluk et al. 1998), LT50 (Brule-Babel and Fowler 1988, Sutka and Snape 1989), field survival, flowering time, and fructan content (Hayes et al. 1993), regulation of drought induced ABA accumulation (Quarrie et al. 1994), and stress tolerance to several minerals (Manyowa and Miller 1991). These seemingly complex groupings of tightly linked genes of diverse function can also be explained by the pleiotropic action of regulators that synchronize multi-factorial physical, biochemical and morphological responses of integrated environmentally-induced genetic systems, i. e., master switches.
The function of cells requires that single genes be expressed at different levels and that different genes be expressed at different stages of development and in different tissues. We know this to be intuitively correct because each cell in our body has the genetic information to produce all our body parts yet growth and development proceeds in an orderly, regulated fashion. Similarly, developmental regulation of low-temperature tolerance gene expression provides for a highly integrated system that allows for maximum biochemical and physiological efficiency. An effective system for regulation of low-temperature tolerance responses in plants would also permit multiple use of adaptive mechanisms thereby allowing the successful plant to maintain its competitive advantage by optimizing its metabolic activities. The vrn1 complex and related developmental regulators on chromosome 5A of wheat appear to be an example of this type of highly integrated system (Fowler and Limin 1997).
Role of transcriptional factors and promoters
Genes are expressed through the transcription of DNA sequences to produce mRNAs, which in turn provide the messages that are translated into proteins. Gene expression is driven by a promoter, which is a DNA sequence generally found at the front of the protein coding region of the gene. The promoter determines where, when, and to what extent the gene is expressed. Regulatory elements of various kinds that act as binding sites for unique transcription factors are found throughout these promoters. Regulatory elements can activate or repress transcription, depending on the specific situation. The transcriptional machinery involves several protein factors that interact within the promoter to determine the characteristics of gene expression including where and when the gene is expressed. Promoter sequences found further upstream will also attract specific proteins known as trans-acting or transcription factors, which will activate or repress the transcription machinery in the appropriate cells.
Extrapolation of the present limited knowledge of the genetics of cold hardening in plants suggests that low-temperature tolerance gene expression may be controlled by regulatory elements that act as promoter binding sites for transcription factors that activate the gene. The known low-temperature induced genes carry the same regulatory sequences and they are likely regulated by the same transcriptional factors (Jaglo-Ottosen et al. 1998). The transcription factors vary with environmental conditions and developmental stage and modify gene expression through a combination of transcriptional activation, amplification, repression, and integration that is difficult to predict (Wray 1998). For example, activation of the low-temperature induced promoter of cold regulated Arabidopsis genes (cor15a) can be overridden by developmental cues (Baker et al. 1994) suggesting that developmental genes have an important low-temperature tolerance regulatory role in determining the level of cold-induced gene expression. Involvement of enhancer elements as well as negative and positive regulatory regions for transcriptional regulation in the promoter of low-temperature induced genes have been reported (Ouellet et al. 1998). Multiple DNA binding (repressor) proteins have been associated with promoter regions of plants grown in warm non-acclimating conditions, but these proteins were absent from the promoter in low-temperature acclimated plants (Vasquez-Tello et al. 1998) indicating a temperature regulated system. These observations point to a system of developmental and pleiotropic regulation that is responsive to temperature cues. They also suggest developmental genes may act as master switches that are capable of regulating the level of expression of cold hardiness genes.
Given the close associations between the vernalization genes and the low-temperature genes, it is possible that vernalization and low-temperature responses are interrelated (Fowler et al. 1996b) and the vernalization (vrn) genes located on chromosome 5A may be pleiotropic, regulating both phenological development and the expression of low-temperature tolerance in wheat (Brule-Babel and Fowler 1988). Molecular studies designed to investigate these interactions have demonstrated that the regulatory influence exerted by the vrn complex over low-temperature induced structural gene (Wcs120 and Wcor410) expression occurs at the transcriptional level in winter cereals (Fowler et al. 1996a). The wheat group 5 chromosomes, which carry the Vrn genes, have also been found to regulate the expression of a least four low-temperature regulated gene families correlated with low-temperature tolerance (Sarhan and Danyluk 1998). These same chromosomes (particularly 5A) induce higher levels of expression in many low-temperature induced genes that are dispersed across all 3 wheat genomes (Limin et al. 1997, Danyluk et al. 1998) indicating a single transcriptional activator on chromosome 5A is able to target the low-temperature induced genes. Studies have provided further evidence for regulation of dehydrin genes by group 5 chromosomes. In fact, it has been suggested (Campbell and Close 1997) that barley Sh2 (=Vrn1 homoeoallelic wheat series) may be a dehydrin gene with major regulatory and developmental influence as a result of autoregulatory activity on other dehydrin genes.
The above observations indicate that genes located on chromosome 5A act as a master switch that plays the role of both developmental regulator and transcriptional activator of low-temperature induced genes located on group 6 chromosomes (Figure 7). The master switch theory accommodates the fact that group 5 chromosomes in wheat, and their homoeologous chromosomes in barley and rye, carry both the strongest vernalization genes and the most important low-temperature tolerance genes (Fowler et al. 1999). It also explains why so many of the stress and low-temperature induced characters appear to be regulated by genes associated with vernalization genes on chromosome 5 and accounts for the often overlooked fact that vernalization and low-temperature acclimation have similar temperature ranges for induction.
Interspecific gene transfers and transformation
While much is known about plant low-temperature response, the maximum cold hardiness potential of most crops has reached a stubborn plateau that has not been breached for decades. In fact, all the efforts of modern science have been unable to produce the super hardy cultivars needed to expand winter crop production into regions requiring a level of cultivar low-temperature tolerance superior to that found in the land races selected by early farmers. The search for superior low-temperature tolerance genes has been expanded to include attempts at interspecific and intergeneric transfers. There are considerable differences in the maximum low-temperature tolerances found in different winter cereals (Fowler et al. 1997, Fowler and Carles 1979, Limin and Fowler 1981) and the possibility that genes can be transferred between species to increase the genetic variability available to winter cereal breeding programs has been explored. However, these attempts have done little more than demonstrate the difficulties that must be overcome before the full potential of superior species-specific cold-tolerance gene expression can be captured through interspecific gene transfers in plant breeding programs.
Rye is the cereal most responsive to low-temperature induction, producing a more rapid rate of cold acclimation and up-regulating low-temperature associated genes to higher levels than other species (Fowler et al. 1996a, 1996b). Unfortunately, while the structural genes within the Triticeae have a high degree of homology and the regulation of low-temperature tolerance is operational across genomes, we have not been able to successfully exploit the superior low-temperature tolerance genes of rye for cultivar improvement in related cereal species. The superior low-temperature tolerance of rye was suppressed when combined in tetraploid (Limin et al. 1985) and hexaploid (Dvorak and Fowler 1978) wheat backgrounds. Artificially synthesized ABD genome hexaploid wheat (Limin and Fowler 1982) also demonstrated the nonadditivity of closely related genomic systems. Further investigation of low-temperature tolerance gene expression in hybrids among Triticeae species (Limin and Fowler 1988, 1989) lead to the conclusion that chromosome dosage or ratios influence low-temperature tolerance by shifting competitively balanced systems toward the parent with the greatest chromosome number. Molecular investigations of these hybrids has subsequently revealed that highly conserved and coordinately regulated low-temperature induced gene families of both species are expressed in interspecific crosses (Limin et al. 1995). However, these genes were not expressed independently and the degree of low-temperature gene expression in these interspecific crosses was regulated at the transcriptional level by the higher ploidy parent.
These observations indicate that, before we can successfully exploit alien genetic variability for low-temperature tolerance, we must first acquire a greater understanding of the complex genetic mechanisms that plants have evolved for the efficient integration of low-temperature responses into the daily processes of survival, growth, and reproduction. As emphasized earlier, just about every morphological, physiological, and biochemical characteristic that can be measured in the plant changes during low-temperature acclimation. This observation in itself suggests that low-temperature acclimation involves a large number of genes. Threshold induction temperatures, time-temperature relationships for acclimation and deacclimation, effectiveness of regulators, morphological adjustments to changes in light and temperature, and factors that influence the plants transition from the vegetative to the reproductive phase all appear to have an important influence on low-temperature gene regulation in this system. Clearly, low-temperature tolerance gene expression is influenced not only by environment, but also by the pleiotropic effects of other genes. Until biotechnology tools became available it was difficult to separate the genes responsible for low-temperature acclimation and cold tolerance from those associated with metabolic adjustments to low temperature. Molecular genetics has provided researchers with the ability to separate out cause-and-effect relationships and an opportunity to explain some of the apparent contradictions that exist in the low-temperature tolerance literature. This area has been progressing rapidly and we have moved beyond molecular mapping and protein accumulation studies into functional genomics and a more in depth consideration of cause-and-effect relationships with an overall objective of achieving a clearer understanding of the genetic cascade controlling gene expression during acclimation. These investigations have started with a search for the principle mechanisms that winter cereals use to regulate low-temperature gene expression.
Breeding for Resistance to Freezing Stress
A large research effort has been concentrated on solving the mysteries of low-temperature tolerance in plants. Some progress has been made, but the impact of this research on plant breeding has been minimal. While plant breeders have successfully maintained low-temperature tolerance levels of crop plants within established production areas, attempts to produce super-hardy cultivars using the existing genetic variability within species have met with limited success in this century. The primary reasons for this lack of success includes. a) Exploitable genetic variability for low-temperature tolerance has been largely exhausted within the gene pools of most species. b) A large number of genes with small effects and complex interactions are assumed to determine the phenotypic expression of this character making selection difficult. Although most studies indicate that low-temperature tolerance within species is controlled mainly by genes with additive effects; recessive, partial dominant, and overdominant control have also been reported. c) Current methodology for measuring low temperature tolerance gives poor resolution of small phenotypic differences. d) Measures of low-temperature tolerance lack the precision for single plant analysis and many are destructive making selection procedures complicated. e) Poor expression of low-temperature tolerance genes in alien genetic backgrounds has prevented the expansion of gene pools through interspecific and intergeneric transfers e.g., the superior low-temperature tolerance of rye is suppressed in a wheat background.
The ability to acclimate or avoid low-temperature stress varies among species and stages of crop growth. As a consequence, each plant-breeding situation is unique and it is impossible to design a single approach that will satisfy the requirements of all low-temperature tolerance breeding programs. For example, the criteria used to select for low-temperature tolerance are different for actively growing plants that must survive short periods of late spring frost compared to plants that must withstand exposure to long periods of below freezing winter temperatures. When pure, water can supercool or remain unfrozen to its homogeneous nucleation point of approximately -40oC and, when environmental sources of ice nucleation are absent, supercooling is an important mechanism that determines the level of damage in actively growing plants. Stage of plant development and physical barriers such as the stem nodes, rachis and rachilla that slow ice nucleation and the spread of ice crystals are some of the factors that determine the impact of supercooling on the low-temperature tolerance of tender tissue. Supercooling is also an important survival mechanism for certain cells in woody plants that must survive very low winter temperatures and thermal analysis to detect exotherms resulting from nucleation of supercooled cells have been used to identify differences in the low-temperature tolerance in these species. However, while supercooling is an important low-temperature tolerance mechanism in these instances, winter survival in most plant tissues depends on an ability to accommodate freezing temperatures and prevent damage to the cytoplasm by slowly forming ice crystals in intercellular spaces. Consequently, slow freezing rates and ice nucleation to avoid supercooling are important considerations when conducting freezing tests on most plants that have the ability to low-temperature acclimate. These few examples have been given to emphasize the need for plant breeders to have a thorough understanding of the low-temperature tolerance problems they are addressing. This short introduction also raises a few basic questions that need to be answered to ensure the effectiveness of a low-temperature tolerance-breeding program.
1) What are the climatic conditions of the region that the breeding program targets?
2) What are the critical stress periods for the species under selection?
3) What low-temperature tolerance or avoidance mechanisms are employed and what are the sources of genetic variability in the species under selection?
4) What are the most appropriate methods for identifying superior individuals in breeding populations? Can artificial environments that accurately reproduce the critical stresses experienced in nature be created to facilitate selection programs? Are there low-temperature tolerance prediction tests available that have been field validated for the target region? Do the prediction tests provide measures of field reaction that are accurate enough to be effective as selection tools?
5) Are the necessary resources available to properly implement the selected breeding strategy?
A detailed consideration of breeding strategies for different species in different environments is beyond the scope of this paper. Consequently, the remainder of this discussion will focus primarily on cereal crops as an example of the complex problems encountered when breeding for low-temperature tolerance.
Prediction and controlled-freeze tests
The limitations inherent in field survival trials have provided a continuing incentive for researchers to search for rapid, more efficient methods of predicting low-temperature tolerance. Ideally, a low-temperature tolerance screen should be simple, rapid, repeatable, and non-destructive. It should also provide an accurate, precise measure of cold hardiness potential on single plants. The many changes that occur in morphological, biochemical, and physiological characters during low-temperature acclimation have provided fertile ground in the search for prediction tests and differences in several characters, such as plant erectness in winter cereals, tissue water content, cell size, etc., have been shown to be highly correlated with freezing tolerance (Fowler et al. 1981, Limin and Fowler 2000). Use of these characters as screens satisfies many of the criteria listed above. However, while prediction tests have been used effectively for preliminary low-temperature tolerance selection in breeding programs, high experimental errors limit their usefulness in identifying small differences that are of practical concern to plant breeders, especially where breeding programs target high stress regions.
Controlled-freeze tests on plants that have been cold acclimated in artificial environments are routinely used to measure low-temperature tolerance in physiological and genetic studies. As indicated earlier, rates of freezing and thawing and recovery conditions must reflect the conditions that the plant will experience in nature for controlled-freeze tests to have predictive value. Well-designed controlled-freeze tests that employ a single minimum temperature provide a resolution of low-temperature tolerance differences similar to field trials and the same level of caution must be exercised in interpreting results from these two methods. Poor reproduction of cold acclimation rates in repeat experiments makes selection of critical minimum temperatures in controlled-freeze tests almost as difficult as the identification of field sites that yield a high frequency of injury in the target stress range. A series of test temperatures that establish the LT50 (minimum temperature that 50 percent of plants survive) of populations reduces the difficulties associated with identifying critical selection temperatures. Measures of LT50 provide the highest precision and heritability of all low-temperature tolerance prediction tests (Fowler et al. 1981) but, they require a sample of plants from a homogeneous population that can be tested using a series of test temperatures or times. This restricts LT50 measurements to pure lines and limits their usefulness in plant breeding programs that are normally dealing with segregating populations. Increased availability of practical methods for doubled haploid production and asexual propagation, which provide means for quickly producing homogeneous populations, expand the opportunities to use LT50 estimates for selection in plant breeding programs.
The cold tolerance of winter cereal crowns is reduced by prolonged exposure to sub-lethal temperatures and, as a consequence, both temperature and exposure time are important variables in controlled-freeze test procedures. The predicable relationship between time and temperature (Equation 1) has meant that survival time at a constant below freezing temperature (Figure 5; in Impact of Cold Stress) can also be used to identify differences in plant low-temperature tolerance (Thomas et al. 1988).
When combined with the use of controlled environments to simulate conditions for low-temperature acclimation, controlled-freeze-tests provide for greater flexibility in the timing of experiments than field trials. However, while controlled environments should theoretically allow for more rigid control of experimental conditions, comparative studies have shown that field trials usually provide more repeatable results and have lower experimental errors (Fowler et al. 1981). Consequently, regardless of available resources, field screens are routinely used to provide the final measure of winter survival potential in most breeding programs.
Marker Assisted Selection
The selection for complex genetic traits, such as low-temperature tolerance, can be simplified in plant breeding programs when linked qualitative markers are identified. In wheat, the identification of a dominant gene(s) affecting low-temperature tolerance that is closely associated with the vernalization (vrn1) (Brule-Babel and Fowler 1988, Sutka and Snape 1989) and prostrate growth habit (Roberts 1990) genes on chromosome 5A has proven to be an important exception to the additive gene action rule. Within the Triticeae, the genes for vernalization are found on the 4th, 5th, and 1st group chromosomes (McIntosh et at. 1998) while the 4th and 5th group chromosomes most commonly associated with low-temperature tolerance (Sutka 1981, Law and Jenkins 1970). Subsequent work with barley has identified a linkage between low-temperature tolerance and vernalization genes on chromosome 7 (Hayes et al. 1993), which is homeologous with the 5th chromosome group in wheat. These linkages have provided valuable phenotypic markers for the investigation of low-temperature tolerance in Gramineae.
The developing science of biotechnology has provided an ever-increasing number of molecular markers that can be used to assist selection in plant breeding programs. However, marker assisted selection requires a detailed linkage map and there is still a dearth of markers for low-temperature tolerance genes. For example, molecular markers in the cereals have been limited to the chromosome regions associated with the homoeoallelic genes Vrn1 in wheat (Galiba et al. 1995), Sp1 in rye (Plaschke et al. 1993) and Sh2 in barley (Laurie et al. 1995), regions that were previously known to have a significant influence on plant cold hardiness. However, with as many as 15 out of 21 chromosomes in wheat having an influence on low-temperature tolerance (Stushnoff et al. 1984), there is still considerable work to be done before the full potential of mapping assisted selection can be realized for this character in most cereal breeding programs.
Advances in biotechnology have provided additional opportunities for plant breeders to expand their attack on the winter-hardiness barrier that has frustrated them for so long. The large number of cold-induced genes associated with low-temperature tolerance and their products that have been isolated in recent years have provided us with a snapshot of these opportunities. For example, antibodies raised against WCS120 proteins produced by low-temperature induced Triticeae genes (Houde et al. 1992) have provided an immediate practical opportunity to simplify cold hardiness selection procedures in breeding programs. Low-temperature induced expression of this gene family in wheat, as measured by densitometry scanning of Western blots, closely follows changes in LT50 thereby providing a direct means of quantifying phenotypic differences in low-temperature tolerance of cereals (Fowler et al. 1996a).
The rapidly expanding area of functional genomics offers even larger opportunities for the understanding and manipulation of complex genetic systems. As expected in the Triticeae, these investigations have focused on the major low-temperature tolerance genes associated with the vernalization loci. Identification of the main genetic pathways responsible for low-temperature gene expression in one species should provide a guide to charting the routes and establishing the regulation of low-temperature gene expression in other species, especially those that are closely related. Molecular procedures that facilitate the analyses of genome structure and organization for purposes of comparative molecular mapping should allow us to further expand our ability to simultaneously exploit discoveries in a wide range of species. Transformation studies have demonstrated that some of the low-temperature induced genes and their transcriptional factors may be exploited to improve the low-temperature tolerance of tender genotypes (McKersie and Bowley 1998, Kasuga et al. 1999, Thomashow 1999). Certainly, we now have the tools to greatly improve our ability to breed for low-temperature tolerance in plants. However, biotechnology has also brought the pharmaceutical model to plant breeding, which includes patents and expanded intellectual property rights that have greatly restricted plant breeders’ freedom to operate. These changes have not been unopposed and the full potential for biotechnology to revolutionize the plant breeding world will have to wait for the new order to evolve a more widely accepted and workable plant breeding environment.
Field Selection
In spite of the opportunities offered by cold-hardiness indicators, controlled-freeze testing, and molecular markers, most of today’s winter cereal breeding programs still rely heavily on field screening as the final measure of plant winter survival potential. Field-testing is simple, inexpensive, and does not require access to specialized facilities or co-operating programs with conflicting priorities. Unfortunately, the opportunity for selection in field trials only occurs once a year, winters that provide critical selection temperatures usually occur infrequently, and non-uniform stress levels due to variable snow cover and other environmental factors often result in high experimental errors that reduce within-trial selection efficiency.
Where adequate resources are available, a number of measures can be implemented to increase the opportunity for effective field selection for low-temperature tolerance. The frequency of test winters and degree of stress can often be increased by growing trials at or outside the margin of the target production region, thereby increasing the opportunity for selection and the level of selection pressure (Fowler et al. 1993). Where low-temperature responses have been characterized in detail, computer simulation models and historical weather records may be used to assist in the identification of sites with a high probability of low-temperature stress in the desired range (Savdie et al. 1991). Methods that control variation in snow cover may be employed to reduce experimental errors and increase the probability of differential injury among breeding lines of plant species with critical meristems near the soil surface. The inclusion of cultivars with known low-temperature tolerance as reference plots to monitor stress levels in trials and the use of a moving average in data analyses provide further opportunities to a) adjust for non-uniform levels of stress, b) maximize the information gleaned from trials with low levels of differential injury, and c) pool results from different trials. The Field Survival Index (FSI) is an example of how this approach has been used to obtain objective measures of low-temperature tolerance in wheat and rye (Fowler et al. 1981).
Developmental Regulation of Low-Temperature Tolerance
Because perennials survive for several years, the staging and regulation of their vegetative/reproductive changes are much more complex than are those of summer and winter annuals. In many cases both vegetative and fully functional reproductive structures must survive the winter, which greatly increases the range of injuries that affect different parts of the plant and the complexity of breeding for low-temperature tolerance in perennials. The biochemical, physiological, and morphological changes associated with low-temperature tolerance clearly interfere with active growth and to be successful a plant must be programmed to recognize and respond to the environmental cues that signal seasonal changes. For these reasons, low-temperature tolerance in perennials normally follows a yearly pattern of environmental cues that include photoperiod and temperature changes. These cues permit the plant to anticipate periods of stress while optimizing its ability to take advantage of favourable periods for growth and reproduction. Consequently, a large part of the plant breeder’s task involves selection for the complex genetic mechanisms that plants have evolved for the efficient integration of low-temperature responses into the daily processes of survival, growth, and reproduction.
In general, the longer the plant life cycle the more complex and poorly understood is the low-temperature response mechanism. However, while tree breeders have some of the longest living plants to deal with, they have been much more conscious of the linkage between phenological development and low-temperature response in their selection programs than breeders working on species with shorter life cycles. For example, cereal breeders normally restrict the connection between phenological development and low-temperature tolerance to a consideration of spring and winter growth habit. However, there is strong and growing evidence of close links between the up-regulation of low-temperature tolerance genes and vernalization requirement and photoperiod sensitivity in cereals. For example, cereals with a vernalization requirement or short day photoperiod sensitivity acclimate upon exposure to low-temperature until the point of transition to the reproductive stage after which there is a loss in low-temperature tolerance (Fowler et al. 1996a, Mahfoozi et al. 2000). Consequently, the point of transition to the reproductive stage is pivotal in the expression of low-temperature tolerance genes. This interaction also makes all low-temperature tolerance associated characters or genes appear to be associated with developmental genes (Fowler et al. 1999) and explains the pleiotropic effects (growth habit and low-temperature tolerance) attributed to developmental genes like vrn1 in wheat (Brule-Babel and Fowler 1988, Sutka and Snape 1989, Roberts 1990).
The linkage of low-temperature tolerance expression to phenological development adapts the plant to the environment for which it was selected or in which it evolved. For example, a high level of low-temperature tolerance is no longer required after the onset of warm conditions in the spring when rapid growth and reproduction begin. Consequently, satisfaction of dormancy, vernalization, and photoperiod requirement results in a decline in low-temperature tolerance of over-wintering plants. In fact, for species adapted to regions with long, mild winters, a high level of freezing tolerance is often less important than a rigorous photoperiod, dormancy, or vernalization requirement that prevents plants from entering the extremely cold-sensitive reproductive growth stage until the risk of low-temperature damage has passed. Consequently, the evolution of, and selection for, genetic options that permit extensive modification of thermosensitive metabolic processes and critical structural components should not come as a surprise, especially in winter annual and perennial plants that must adapt to a wide range of seasonal challenges. It is this genetic system and its regulation and complex interaction with the environment that makes breeding for low-temperature tolerance a continuing challenge.
Crop Management
The availability of highly adapted cultivars with superior low-temperature tolerance is considered a prerequisite for successful crop production in many areas of the world. However, even the hardiest cultivars can be damaged by low-temperature if proper attention is not paid to management practices, especially in regions of marginal adaptation. Conversely, even the best management systems will not be successful unless highly adapted cultivars are available for production. In other words, genotype establishes crop potential and proper management allows the farmer/grower to optimize this potential.
The role of crop management
Plant survival and economic production on fringes of regions of adaptation for many species has required the use of low-temperature avoidance systems and refined management techniques. For example, the root system is the most susceptible part of the plant to low temperature damage. Use of hardy rootstocks and protective mulches are examples of how orchard managers and gardeners have exploited this knowledge to over winter tender genetic stocks of economic and ornamental value. Field location is also a particularly important consideration in successful orchard and garden management. Protective techniques that include the use of windbreaks, snow trapping, mulches, and transparent covers have a moderating effect that help plants avoid low temperature extremes. In addition to providing insulation, protective covers help the plant avoid large temperature fluctuations and stresses due to alternate freezing and thawing. Similarly, the shading effects of snow cover and other barriers protect evergreens from winter burn and tree trunks from sun scald and desiccation injury. Heaters, sprinklers, and wind machines are regularly used to protect tender plant tissues from damage due to temperatures in the -2 to -5oC range in high value commercial orchards. Similar methods have been investigated for mid winter protection in regions where temperatures are much colder, but success under these conditions requires sophisticated weather prediction and crop monitoring systems.
Light is important as an energy source and an environmental cue. Consequently, the timing and method of pruning and training are important management tools that affect winter hardiness through their influence on shading, the initiation and differentiation of vegetative and floral meristem, and the general health of the plant. Water supply affects growth, tissue water content, and the cooling rate of the immediate environment. Excessive water on frozen soils can also result in damage due to ice encasement.
Diseases can affect the health of the plant and its general ability to tolerate low-temperature stress. Similarly, proper nutrient balance is essential for the production of healthy vigorous plants and deficiencies can be expected to have an adverse effect on the low-temperature tolerance. Deficiencies and excesses of a number of elements have been studied with mixed results that are probably related to the size of the deficiency or excess of the element under study. High levels of salts associated with soil salinity have been shown to reduce the winter hardiness of some plants. Excessive nitrogen fertilization that stimulates luxury growth prior to plant low-temperature acclimation has been reported to prevent full expression of cold-hardiness potential while corrections of deficiencies in phosphorous and potassium are generally associated with increased cold hardiness.
As emphasized earlier, plants have evolved mechanisms that allow them to anticipate seasonal weather changes. Therefore, successful management of winter crops requires an understanding of plant development, growth cycles and the mechanisms used to survive periods of low-temperature stress. An understanding of how plants respond to low-temperature stress at different growth stages can also assist in the assessment of crop condition and production potential throughout the growing season.
Management can succeed where genetics fails
The recent expansion of the western Canadian winter wheat production area is an example of how management practices that modify the plant microenvironment can be used to reduce the limitations imposed by low temperature (see http://www.usask.ca/agriculture/plantsci/winter_cereals). The lack of super-hardy cultivars limited winter wheat production in this region when conventional production systems were employed, i.e., seeding into a tilled seedbed. However, field studies demonstrated that only 8 to 10 cm of unpacked snow was sufficient to maintain soil temperatures above the minimum survival temperatures of critical crown tissue throughout the winter. This knowledge stimulated efforts to develop snow-trapping techniques and it was quickly shown that direct seeding into standing stubble (no-till) from a previous crop provided an ideal means for maintaining uniform snow cover of this depth. Success with this management system has allowed farmers to extend the winter wheat production on the North American Great Plains north and east from a small area along the Canada/USA border in southern Alberta to include the entire western Canadian prairies. This region, which contains 85 percent of the arable land in Canada, has one of the coldest climates for crop production of any large agricultural region in the world. While success in establishing winter wheat as a viable cropping option in western Canada provides an excellent example of the important role that crop management can play in the mitigation of low-temperature stress, the struggle to achieve this success also emphasizes the need for a holistic approach to management problems.
The production of no-till winter wheat is straightforward and simple, but it requires the use of management practices different from those commonly employed by most prairie farmers. In western Canada, no-till winter wheat is seeded into standing stubble from a previous crop between the end of August and the middle of September, and harvested early the following August. Because of the short growing season in this region and the requirement for standing stubble, winter wheat is better suited to rotations that include early maturing spring crops. Consequently, winter wheat growers must start planning for next year’s crop well before this year’s crop is harvested. This necessitates a production schedule that considers management decisions over a period of two or more crop years. This usually means a reassessment of the entire crop rotation and harvesting operation. Rotations have to be planned to include early maturing crops, thereby ensuring that standing stubble is available at an early date. Direct seeding equipment is a necessary component of this package and most farmers have been reluctant to invest in seeding equipment solely for the production of winter wheat. As a consequence, the successful adoption of winter wheat has been closely linked to the level of acceptance of no-till spring crop-production systems. This linkage also means that seeding equipment must be selected carefully to ensure it provides the flexibility required to direct seed a wide variety of crops under a wide range of conditions.
Plant establishment is the critical step in the no-till winter wheat production system. Successful plant establishment has required the acquisition of special management skills and the placement of a high priority on stubble management and the seeding operation. Seeding date and depth both have a large influence on the degree of success that can be achieved in the production of winter wheat in western Canada. Winter wheat over-winters as a seedling and in order to attain maximum cold tolerance and to provide optimum energy reserves for the following spring, healthy vigorous plants must be established before the soil freezes in November for the winter. However, seeding too early can result in excessive fall growth and plants that are less resistant to winter injury and disease. Plants that enter the winter with well-developed crowns (Figure 2 in Impact of Cold Stress – area at the base of the shoot from which secondary roots develop) are most desirable and late dates of seeding usually result in poorly established plants that have lower winter-survival potential. Deep seeding also results in delayed emergence and weak plants that are more susceptible to damage from winter stresses. In this instance, a difference in seeding depth of 5 cm compared to 2.5 cm has meant the difference between a crop that has been winter killed and a healthy crop in the spring.
Many of the soils in western Canada are deficient in phosphorous and both deficiencies and excesses of this element have been shown to reduce the winter-survival potential of winter wheat. However, phosphorus may not have a direct influence on the winter hardiness of the plant. Rather, when deficiencies are corrected, it appears to act by improving root growth and spring recovery from winter injury.
The results of field trials have demonstrated that plant-available soil-nitrogen level does not normally affect the winter-hardiness potential of wheat unless the nitrogen has been applied in the seed-row at the time of planting. Urea (46-0-0) and ammonium nitrate (34-0-0) are the two most common N forms that are seed placed and both can reduce seedling number and size, especially when the soil is dry at seeding. The effect of seed-placed urea is more insidious and damage is usually less of a problem with ammonium nitrate. Also, the concentration of fertilizer immediately adjacent to the seed is dependent upon fertilizer rate, row spacing of the drill, and seed row opener design. High rates of phosphate fertilizer will not offset the effect that seed-row banded nitrogen fertilizer has in reducing winter hardiness. Placement of nitrogen fertilizers a minimum distance of one 2.5 cm from the seed will minimize seedling damage and this has led to considerable recent research on “side-banding” seeding equipment and other fertilizer placement options.
The management practices considered above all have a direct influence on plant establishment and the ability of a cultivar to realize its full winter hardiness potential. These variables are all under the direct control of the producer emphasising the important role that management skills play in the successful production of winter wheat in western Canada. Similar examples can be found for many other crops stressing the need to understand genotype x environment interactions and the important role of crop management when dealing with crop production in high stress environments. Perhaps of equal significance in lower stress regions, is the fact that improved management techniques have allowed plant breeders to reduce their emphasis on low-temperature tolerance and focus a larger proportion of their resources on breeding for other characters of economic concern.
References
Baker, S.S., K.S. Wilhelm and M.F. Thomashow. 1994. The 5′-region of Arabidopsis thaliana cor15a has cis-acting elements that confer cold-, drought- and ABA-regulated gene expression. Plant Mol. Biol. 24:701-713.
Blum, A. 1988. Plant breeding for stress environments. CRC Press, Inc., Boca Raton, Florida.
Brule-Babel, A.L. and D.B. Fowler. 1988. Genetic control of cold hardiness and vernalization requirement in winter wheat. Crop. Sci. 28:879-884.
Brule-Babel, A.L. and D.B. Fowler. 1989. Genetic control of cold hardiness and vernalization requirement in rye. Genome 32:19-23.
Campbell, S.A. and T.J. Close. 1997. Dehydrins: genes, proteins, and associations with phenotypic traits. New Phytol. 137(1):61-74.
Christiansen, M. N. and J. B. St. John. 1981. The nature of chilling injury and its resistance in plants. pp.1-16. In C. R. Olien and M. N. Smith (eds.) Analysis and improvement of plant cold hardiness. CRC Press Inc. Boca Raton U. S. A.
Close, T. J. 1996. Dehydrins: Emergence of a biochemical role of a family of plant dehydration proteins. Physiologia Plantarum 97:795-803.
Close, T. J. 1997. Dehydrins: A commonalty in the response of plants to dehydration and low temperature. Physiologia Plantarum 100:291-296.
Danyluk, J., A. Perron, M. Houde, A.E. Limin, D.B. Fowler, N. Benhamou and F. Sarhan. 1998. Accumulation of an acidic dehydrin in the vicinity of the plasma membrane during cold acclimation of wheat. The Plant Cell 10:623-638.
DeSilva, N.S. 1978. Phospholipid and fatty acid metabolism in relation to hardiness and vernalization in wheat during low temperature adaptation to growth. Z.Pflanzenphysiol. Bd. 86:313-322.
Dvorak, J. and D.B. Fowler. 1978. Cold hardiness potential of triticale and tetraploid rye. Crop Sci. 17:477-478.
Fowler, D.B. and R.J. Carles. 1979. Growth, development, and cold tolerance of fall-acclimated cereal grains. Crop. Sci. 19:915-922.
Fowler, D.B. and A.E. Limin. 1997. Breeding for winter hardiness in cereals. Acta Agron. Hungarica. 45:301-309.
Fowler, D.B., L.P. Chauvin, A.E. Limin and F.Sarhan. 1996a. The regulatory role of vernalization in the expression of low-temperature induced genes in wheat and rye. Theor. Appl. Genet. 93:554-559.
Fowler, D.B., L.V. Gusta and N.J. Tyler 1981. Selection for winter-hardiness in wheat. III. Screening methods. Crop Sci. 21:896-901.
Fowler, D.B., A.E. Limin and J.T. Ritchie. 1999. Low-temperature tolerance in cereals: model and genetic interpretation. Crop Sci. 39:626-633.
Fowler, D.B., A.E. Limin, A.J. Robertson and L.V.Gusta. 1993. Breeding for low-temperature tolerance in field crops. Int. Crop Sci.1:357-362.
Fowler, D.B., A.E. Limin, S-Y. Wang and R.W. Ward. 1996b. Relationship between low-temperature tolerance and vernalization response in wheat and rye. Can. J. Plant Sci. 76:37-42.
Galiba, G., I. Kerepesi, J.W. Snape and J. Sutka. 1997. Location of a gene regulating cold-induced carbohydrate production on chromosome 5A of wheat. Theor. Appl. Genet. 95:265-270.
Galiba, G., R. Tuberosa, G. Kocsy and J. Sutka. 1993. Involvement of chromosome 5A and 5D in cold-induced abscisic acid accumulation in and frost tolerance of wheat calli. Plant Breeding 110:237-242.
Galiba, G., S.A. Quarrie, J. Sutka, A. Morgounov and J.W. Snape. 1995. RFLP mapping of the vernalization (Vrn1) and frost resistance (Fr1) genes on chromosome 5A of wheat. Theor. Appl. Genet. 90:1174-1179.
George, M.F., Becwar, M.R. and Burke, M.J. 1982. Freezing avoidance by deep undercooling of tissue water in winter-hardy plants. Cryobiology. 19:628-639.
Grafius, J.E. 1981. Breeding for winter hardiness. p. 161-174. In C.R. Olien, and M.N. Smith (eds.) Analysis and improvement of plant cold hardiness. CRC Press, Boca Raton, FL.
Griffith, M., M. Antikainen, W-C. Hon, K. Pihakaski-Maunsbach, X-M. Yu, J.U. Chun, and D.S.C. Yang. 1997. Antifreeze proteins in winter rye. Physiol. Plant. 100:327-332.
Hayes, P.M., T. Blake, T.H.H. Chen, S. Tragoonrung, F. Chen, A. Pan, and B. Lui. 1993. Quantitative trait loci on barley (Hordeum vulgare L.) chromosome 7 associated with components of winter hardiness. Genome. 36:66-71.
Houde, M., R. Dhindsa and F. Sarhan. 1992. A molecular marker to select for freezing tolerance in Gramineae. Mol. Gen. Genet. 234:43-48.
Jaglo-Ottosen, K. R., S. J. Gilmour, D. G. Zarka, O. Schabenberger and M.F. Thomashow.
- Arabidopsis CBF1 overexpression induces COR genes and enhances freezing tolerance. Science 280:104-106.
Kasuga, M., Q. Lui, S. Miura, K. Yamaguchi-Shinozaki and K. Shinozaki. 1999. Improving plant drought, salt, and freezing tolerance by gene transfer of a single stress-inducible transcription factor. Nature Biotechnol. 17:287-291.
Kratsch, H.A. and R.R. Wise. 2000. The ultrastructure of chilling stress. Plant, Cell and Environment 23:337-350.
Laurie, D.A., N. Pratchett, J.H. Bezant and J.W. Snape. 1995. RFLP mapping of five major genes and eight quantitative trait loci controlling flowering time in a winter spring barley cross. Genome 38:575-585.
Law, C.N. and G. Jenkins. 1970. A genetic study of cold resistance in wheat. Genet. Res. Camb. 15:197-208.
Levitt, J. 1980. Responses of plants to environmental stress. 2nd edition, Volume I. Chilling, freezing, and high temperature stresses. Academic Press, New York.
Li, P.H. 1989. Mefluidide: a synthetic chemical that protects corn and rice seedlings from chilling injury. pp167-176. In Li P.H. (ed) Low temperature stress physiology. CRC Press Boca Raton U. S. A.
Limin, A.E., J. Danyluk, L.P. Chauvin, D.B. Fowler and F.Sarhan.1997. Chromosome mapping of low-temperature induced Wcs120 family genes and regulation of cold-tolerance expression in wheat. Mol. Gen. Genet. 253:720-727.
Limin, A.E., J. Dvorak and D.B. Fowler. 1985. Cold hardiness in hexaploid wheat. Can. J. Plant Sci. 65:487-490.
Limin, A.E. and D.B. Fowler. 1981. Cold hardiness of some relatives of hexaploid wheat. Can. J. Bot. 59:572-573.
Limin, A.E. and D.B. Fowler. 1982. The expression of cold hardiness in Triticum species amphiploids. Can. J. Genet. Cytol. 26:405-408.
Limin, A.E. and D.B. Fowler. 1988. Cold hardiness expression in interspecific hybrids and amphiploids of the Triticeae. Genome 30:361-365.
Limin, A.E. and D.B. Fowler. 1985. Cold-hardiness response of sequential winter wheat segments to differing temperature regimes. Crop Sci. 25:838-843.
Limin, A.E. and D.B. Fowler. 1989. The influence of cell size and chromosome dosage on cold hardiness expression in the Triticeae. Genome 32:667-671.
Limin, A.E. and D.B. Fowler. 1991. Breeding for cold hardiness in winter wheat: problems, progress and alien gene expression. Field Crops Res. 27:201-218.
Limin, A.E. and D.B. Fowler. 1994. Relationship between guard cell length and cold hardiness in wheat. Can. J. Plant Sci. 74:59-62.
Limin, A.E. and D.B. Fowler. 2000. Morphological and cytological characters associated with low-temperature tolerance in wheat (Triticum aestivum L. em Thell.). Can. J. Plant Sci. 80:687-692
Limin, A.E. and D.B. Fowler. 2001. Inheritance of cell size in wheat (Triticum aestivum L.) and its relationship to the vernalization loci. Theor. Appl. Genet. (In press).
Limin, A.E., M. Houde, L.P. Chauvin, D.B. Fowler and F. Sarhan.1995. Expression of the cold-induced wheat gene Wcs120 and its homologs in related species and interspecific combinations. Genome 38:1023-1031.
Lyons, J. M. 1973. Chilling injury in plants. Ann. Rev. Plant Physiol. 24:445-466.
Mahfoozi, S., A.E. Limin, P.M. Hayes, P. Hucl and D.B. Fowler. 1998. Developmental control of low-temperature tolerance in cereals. Proc. Ninth Inter. Wheat Gen. Sym., Saskatoon, Sask., Can. 4:54-56.
Mahfoozi, S., A.E. Limin and D.B. Fowler. 2000. Influence of photoperiod response on the expression of cold hardiness in wheat and barley. Can. J. Plant. Sci. 80:721-724.
Mahfoozi, S., A.E. Limin and D.B. Fowler. 2001. Influence of Vernalization and Photoperiod Responses on Cold Hardiness in Winter Cereals. Crop Sci. (In press).
Manyowa, N.M. and T.E. Miller. 1991. The genetics of tolerance to high mineral concentrations in the tribe Triticeae – a review and update. Euphytica 57:175-185.
McIntosh, R.A., G.E. Hart, K.M. Devos, M.D. Gale and W.J. Rogers. 1998. Catalogue of gene symbols for wheat. pp 119 -120 Proc. 9th International Wheat Genetics Symposium, University Extension Press, University of Saskatchewan, Saskatoon, SK, Canada.
McKersie, B.D. and Bowley, S.R. 1998. Active oxygen and freezing tolerance in transgenic plants. pp 203-214 Plant Cold Hardiness. P.H. Li and T.H.H. Chen (ed.). Plenum Press. New York.
Nishida, I. and N. Murata. 1996. Chilling sensitivity in plants and cyanobacteria: The crucial contribution of membrane lipids. Annu. Rev. Plant Physiol. Plant Mol. Biol. 47:541-568.
Plaschke, J., A. Borner, D.X. Xie, R.M.D. Koebner, R. Schlegel and M.D. Gale. 1993. RFLP mapping of genes affecting plant height and growth habit in rye. Theor. Appl. Genet. 85:1049-1054.
Ouellet, F., A. Vazquez-Tello and F. Sarhan. 1998. The wheat wcs120 promoter is cold-inducible in both monocotyledonous and dicotyledonous species. FEBS Letters: 423:324.
Quarrie, S.A., M. Gulli, C. Calestani, A. Steed, and N. Marmiroli. 1994. Location of a gene regulating drought-induced abscisic acid production on the long arm of chromosome 5A of wheat. Theor. Appl. Genet. 89:794-800.
Roberts, D.W.A. 1990. Identification of loci on chromosome 5A of wheat involved in control of cold hardiness, vernalization, leaf length, rosette growth habit, and height of hardened plants. Genome 33:247-259.
Sarhan, F. and J. Danyluk. 1998. Engineering cold-tolerant crops – throwing the master switch. Trends in Plant Sci. 3:289-290.
Savdie, I., R. Whitewood, R.L. Raddatz, and D.B. Fowler. 1991. Potential for winter wheat production on the Canadian prairies: a CERES model winterkill risk assessment. Can. J. Plant Sci. 71:21-30.
Stushnoff, C., D.B. Fowler and A. Brule-Babel. 1984. Breeding and selection for resistance to low temperature. pp 115-136. In P.B. Vose (ed.) Plant Breeding – A contemporary basis. Pergamon Press, Oxford.
Sutka, J. 1981. Genetic studies of frost resistance in wheat. Theor. Appl. Genet. 59:145-152.
Sutka, J. and J.W. Snape. 1989. Location of a gene for frost resistance on chromosome 5A of wheat. Euphytica 42:41-44.
Thomas, J.B., G.B. Schaalje and D.W.A. Roberts.1988. Prolonged freezing of dark-hardened seedlings for rating and selection of winter wheats for winter survival ability. Can. J. Plant Sci. 68:47-55.
Thomashow, M.F. 1999. Plant cold acclimation: freezing tolerance genes and regulatory mechanisms. Annu. Rev. Plant Physiol. Plant Mol. Biol. 50:571-599.
Vazquez-Tello, A., F. Ouellet and F. Sarhan. 1998. Low temperature-stimulated phosphorylation regulates the binding of nuclear factors to the promoter of Wcs120, a cold-specific gene in wheat. Mol. Gen. Genet. 257:157-166.
Watson, J.D., N.H. Hopkins, J.W. Roberts, J. Angetsinger Steitz and A.M. Weiner. 1987. Molecular biology of the gene (Vol. 1). Benjamin/Cummings Publ. Co., Inc., Reading, Massachusetts.
Wray, G.A. 1998. Promotor Logic. Science 279:1871-1872.
Yoshida, S., K. Hotsubo, Y. Kawamura, M. Murai and K. Arakawa. 1999. Alterations in intracellular pH in response to low temperature stress. J. Plant Res. 112:225-236.