The Impact of Flooding Stress on Plants and Crops
Prof. Michael B. Jackson, School of Biological Sciences, University of Bristol, UK and Plant Ecophysiology, Faculty of Biology, University of Utrecht, The Netherlands.
INTRODUCTION
The complex and highly developed land-based biology with which we are most familiar, is a relatively new phenomenon in evolutionary terms. Its existence is predicated on a successful invasion from the sea by photosynthetic macrophytes ~ 400 million years ago (Corner, 1964). This gave rise to organisms with the then novel ability to operate photosynthetically in air while securing water and minerals using a non-photosynthetic, foraging root system. Present day representatives of the more than 300,000 plant species presently known to science now occupy almost every terrestrial niche. However, although, their progenitors were aquatic, the land plants derived from them are relatively intolerant of free water in their surroundings, especially if it is slow moving or immobile. The resulting effect is so severe that biochemical and morphological adaptations have emerged many times during evolution (Cook, 1999) to allow a sizeable minority of species to succeed in sporadically or permanently flooded areas on land. These include areas prone to ice encasement (Andrews, 1996). The ability of excess water to damage plants may seem counter-intuitive since water is chemically benign. However, certain physical properties of water, most notably its ability to interfere with free gas exchange, can injure and kill plants when they are totally submerged (Jackson and Ram, 2003) or even when only the soil is waterlogged (Vartapetian and Jackson, 1997).
The non-frozen permanently wet places are known variously as bogs, mires, marshes, fens, peatlands, bottomlands, wetlands etc. These can be natural or man-made, static or flowing, fresh, brackish or salty, seasonal or permanent. They are widespread and contain a highly adapted and characteristic flora that is under threat from drainage, peat extraction and re-development. These destructive activities conflict with an increasing recognition of the considerable economic, ecological, social and amenity value of many wetlands. They have great significance as wildlife sanctuaries, as buffer zones that reduce flooding intensity of surrounding areas and detoxify the drainage water. This recognition gave rise to the Convention on Wetlands, an intergovernmental agreement adopted at Ramsar, Iran on 2 February 1971. Its remit is to work for the protection and wise management of wetlands world-wide. To-date (2003), the Ramsar Convention had 136 signatory countries, with 1287 wetland sites included in the Ramsar List of Wetlands of International Importance.
The extent of more or less permanently wet places is more readily quantified than areas subjected to sporadic waterlogging or deeper flooding. In reality, almost all the land surface becomes flooded at some time. This is true even in deserts, such as those of central Australia. Worldwide, it has been estimated that approximately 10 % of all irrigated farmland suffers from frequent waterlogging, which may decrease crop productivity 20 %. But, in addition, many rainfed regions are also susceptible to temporary flooding. Satellite imaging has the potential to locate these areas of temporarily flooded land but the technique is under-utilized compared with similar work assessing the extent of water deficient soils.
Clearly, stress from flooding has, and remains, a major influence on species distribution worldwide. It can be the dominant determinant of species success in wet areas (Lenssen et al. 2003). Furthermore, along with drought, salinity and mineral deficiency, flooding also has serious economic consequences for productivity of much arable farmland (see later section entitled ‘Waterlogging and crop production’). This review assesses the impact of excess water on plant growth and development and on the underlying biochemistry and molecular biology. Particular attention is given to responses that appear to enhance tolerance or survival. The major part deals with waterlogging of the soil, and its impact on root systems, the aerial shoot and on farm crops. This is preceded by an assessment of the factors that influence the scale and occurrence of waterlogging and submergence.
FACTORS FAVOURING OCCURRENCE OF WATERLOGGING AND SUBMERGENCE
Increasing water input
It is self-evident that episodic waterlogging of the soil or deeper submergence (referred to collectively as flooding when a distinction is not necessary) occur when water enters soil faster than it can drain away under gravity. There is mounting evidence that, in several parts of the World, inputs of water are growing. One cause may be climate change. For example, records show an increased incidence of flooding and rainfall in much of northern and Western Europe during the last century. Modelling studies indicate that this shift to more intense rainstorms in this region may raise both the frequency and magnitude of flooding from river overflow over the next 50 years (Prudhomme C., Jakob, and Svensson, 2002). A factor contributing to increased rainfall will be faster seawater evaporation at the warmer temperatures that, in turn, may produce more rain. Associated with these trends is an increase in sea level predicted to be up to 20 cm over the next half century. This will principally be the result of thermal expansion and melting of polar ice. Greater fluxes of river water resulting from mountain deforestation are also being experienced in many parts of the World, with loss of mountain forest and wetlands playing a major part in heightening peaks of river outflow. The overall outcome is an increasing frequency of flooding of lowland regions such as the lower reaches of the River Rhine in Europe and the Euphrates delta of Bangladesh and West Bengal. These are highly populated areas but also contain much productive farmland where satellite imaging has recorded many major flooding events. While it is damage to human life and property that attracts most media coverage, flash flooding can devastate vegetation of poorly adapted species especially farm crops. In developing countries especially, this threatens the well-being of many people who depend on locally produced food.
Intensive and large-scale irrigation of farmland can also increase the incidence of waterlogging of the soil. Water tables can rise as a result. This is especially likely in heavily irrigated dry regions such as Sindh Province in the Indus valley of Pakistan. Here, 50 to 60 years ago, the water table was 4 m below ground. By 1984, it was less than 1.6 m over most of the irrigated region, the rising water being laden heavily with salts. The resulting environmental catastrophe has led to a multi-million-dollar drainage project (the Left Bank Outfall Drainage Project) of immense scale but bringing with it much controversy and environmental concern. The problem is exacerbated by the flatness of the topography that inevitably slows the rate of lateral drainage.
A third contributory factor can be change of land use. For example, conversion of meadow land to arable farming in Germany since the 1950s, has contributed to increased surface run-off and exacerbated flooding problems elsewhere in the landscape (Van Der Ploeg et al., 2002). Expanding urbanization of the landscape also creates large expanses of non-absorbing hard surface that concentrates rainwater to its periphery via surface run-off or underground drainage systems.
Slow drainage through the soil profile
The duration and severity of flooding or deeper submergence can be influenced not only by the rate of water input but also by the rate of water flow out from the rooting zone and by the water absorbing capacity of the soil. Topography plays an important part in determining the speed of lateral flow within and above the soil. Obviously, it will be slower on the plains than on sloping land. However, the impact of rate of vertical drainage through the soil profile is critical and strongly affected by soil structure, which is highly variable. Soil structure is a complex subject, involving both macro- and micro-structural components. It is well-described in Soils an Introduction (5th edition) by Michael J. Singer and Donald N. Munns. In brief, it can be said that approximately 40 – 60 % of the soil volume is made of solid material (mostly minerals and organic matter) that is permeated by spaces filled with water, with gas or with roots and other living organisms. The total volume of these spaces (pores), the size range of the pores, their interconnectivity, stability and the relative proportions of each size class all have a major impact on how much water is held by the soil and how readily it drains through the profile. Small pores hold water more strongly by capillary forces than do larger ones. Adopting the classification of (Greenland, 1977), interconnected pores with a diameter range larger than 50 μm (transmission pores) drain under gravity. This allows air to enter (critically oxygen) to support aerobic respiration and also gives space for root exploration. Pores with diameters in the range of approximately 50 – 0.5 μm (storage pores) can hold water against the force of gravity but weakly enough for roots to extract it using driving forces of up to -1500 kPa. However, they are not large enough to allow roots to penetrate. Pores smaller than 0.2 μm hold water so strongly that neither gravity nor roots can extract their contents. These are, therefore, permanently filled with water and are termed residual pores. These classifications are summarized in Table 1, which contrasts a sandy loam with a more clayey soil. It is readily apparent that it will take relatively little extra water for a clay soil to become waterlogged from field capacity compared to a sandy loam soil. Pores in clay soils are also unstable when emptied of water. Thus, drainage is not always followed by the entry of air because some pores collapse. A further consideration is the interconnectedness of transmission pores. The ease of movement of water under gravity from pore to pore is quantified as the soil’s hydraulic conductance (mm d-1). The pores of clay soils are less well connected than those of sandier
soils and thus drain more slowly because hydraulic conductance is low. Drainage rates are also affected by a soil’s macro-structure. In clayey soils, the small intrinsic hydraulic conductance can be offset to some extent by a tendency to crack thereby opening up fissures through which water may move readily. Channels formed by earthworms, decayed root axes, also improve the rate of drainage. The tendency for soil particles to form aggregates (crumbs and clods) with relatively wide channels between them also influences drainage rate. On a larger scale, the rate of soil drainage can be affected indirectly by the hydraulic conductivity of the sub-soil. An impermeable layer at sub-soil depth such as that created by the soles of ploughs, or imposed naturally as in so-called duplex soils, can cause saturation and flooding of the topsoil that, in itself, has good drainage properties. In duplex soils, rainwater moving readily through the sandy topsoil layers then encounters an impermeable layer, typically rich in clay that may have been further compacted by the use of heavy agricultural machinery. In wet weather, the outcome is a perched water table that limits rooting depth and saturates much of the overlying soil. In Australia, large areas used for growing wheat and other crops in south west of Western Australia, and in Victoria and Queensland are especially susceptible to flooding by such perched water tables (see below).
SOIL WATERLOGGING
Oxygen shortage and other damaging features of waterlogged soil
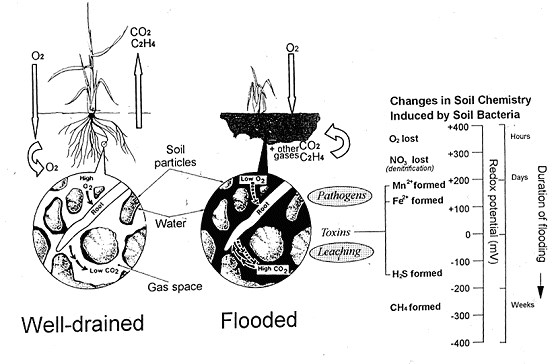
In waterlogged soil, diffusion of gases through soil pores is so strongly inhibited by their water content that it fails to match the needs of growing roots. A slowing of oxygen influx is the principal cause of injury to roots, and the shoots they support (Vartapetian and Jackson, 1997). The maximum amount of oxygen dissolved in the floodwater in equilibrium with the air is a little over 3 % of that in a similar volume of air itself. This small amount is quickly consumed during the early stages of flooding by aerobic micro-organisms and roots (Fig. 2). In addition to imposing oxygen shortage, flooding also impedes the diffusive escape and/or oxidative breakdown of gases such as ethylene (Arshad and Frankenberger, 1990) or carbon dioxide that are produced by roots and soil micro-organisms. This leads to accumulations that can influence root growth and function. For example, accumulated ethylene may slow root extension, while carbon dioxide in the soil can severely damage roots of certain species e.g., soybean (Glycine max) but not rice (Boru et al., 2003). Trapped carbon dioxide may form bicarbonate ions that can accentuate the effect of high lime content, leading to iron unavailability and chlorosis. http://cotton.crc.org.au/Publicat/Agro/Iron.htm. Warm temperatures and ample supplies of organic matter will inevitably accelerate the development of these potentially damaging soil conditions. If root tips survive oxygen shortage per se, they may be injured or killed by subsequent changes in soil biochemistry (Ponnamperuma, 1972). These changes come about because of microbial respiration that utilizes inorganic ions as alternative electron acceptors to oxygen in order to sustain energy generation (Fig. 2). The changes are associated with measurable decreases in redox potential. Facultative anaerobes first chemically reduce nitrate, converting it to nitrite, nitrous oxide and nitrogen gas (denitrification) rendering nitrate unavailable to roots. As the reducing intensity of the soil increases further obligate anaerobes chemically reduce oxides of Mn4+, and Fe3+ to form highly soluble Mn2+ and Fe2+ (Laanbroek, 1990) that may enter roots and interfere with enzyme activities and damage membranes. Ferrous ion toxicity can be a particular problem for rice farming on acidic soils. If flooding is prolonged, further, anaerobic bacteria may then convert SO42- to H2S, a poison of respiratory enzymes and non-respiratory oxidases. Acidic soils that are low in iron are especially likely to contain free and undissociated H2S (Ponnamperuma, 1972). In the most severely reducing soils, methogenic bacteria reduce carbon dioxide to methane. Although the gas is harmless to plants it is second in importance to carbon dioxide as a greenhouse gas contributing to global warming. Rice paddies are globally significant methane sources.
Flooding may also increase the incidence of soil-borne fungal diseases (Yanar, Lipps, and Deep, 1997). Germinating seeds are particularly vulnerable to fungal colonization (e.g., Gliocladium roseum). Infection of alfalfa, vegetables and trees by phytophthora (wilting), pythium (damping-off) and anaerobic bacteria (e.g. Pseudomonas putida) are common problems in practical farming (Walker, 1991). However, it is not always clear whether injury is principally the result of the microbial infection or of the direct effects of flooding (Davison, 1997) and if infection follows rather than precedes injury.
How an absence of oxygen kills root tips?
Although an absence of oxygen is usually fatal to growing root tips, surprisingly small amounts of external oxygen (e.g. 0.006 – 0.01 mol m-3 in solution) are able keep them alive (note: water in equilibrium in air contains approximately 0.25 mol m-3 of oxygen at 25 ºC). Growth arrest and death arise principally because of (a) demand for ATP exceeds the supply (b) self-poisoning by products of anaerobic metabolism. These and related aspects are examined below.
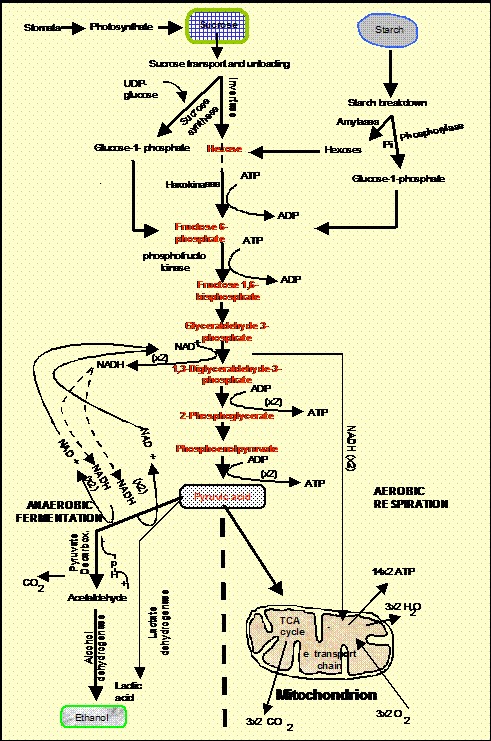
ATP supply and demand
Anaerobic roots generate ATP mainly by glycolysis. This pathway also feeds pyruvic acid into ethanolic fermentation (and also into lactic acid fermentation, especially in the first hours of anoxia before the cytosol acidifies) (Fig. 3). Glycolysis is a cytoplasmic pathway that forms pyruvic acid from glucose, yielding 2 ATPs from each glucose molecule. This is only about 6 % of the ATP generated by mitochondria-based aerobic respiration. Thus, glycolysis is highly inefficient while still requiring a plentiful supply of glucose. It also requires pyridine nucleotide coenzyme in its oxidized form (NAD+). The required NAD+ is generated anaerobically from chemically reduced NADH during ethanolic or lactic acid fermentation (Fig. 3). Unless metabolic processes that consume ATP are simultaneously suppressed, the small yield of ATP in anaerobic cells is insufficient for survival beyond a few hours. Suppression can be brought about if the roots are ‘trained’ beforehand by a few hours of partial oxygen shortage (e.g., 0.04 mol m-3 in solution or 3 % v/v in the air phase) before the supply of oxygen is finally extinguished (Xia, Saglio, and Roberts, 1995). Thus, an inability to restrict ATP utilization to essential life-support processes may be at least as important a reason for death of flooded root tips as slow ATP production. Greenway and Gibbs (2003) conclude that early cell death can only be avoided if the small amounts of available energy are successfully re-diverted to permit synthesis of certain critical ‘anaerobic’ proteins (e.g., alcoholic fermentation enzymes), that support glycolysis and fermentation and help to prevent excessive acidification of the cytoplasm and vacuole (see below) and maintain membrane integrity. A marked decrease of membrane integrity may well be one of the most critical consequences of ATP imbalance for the viability of root cells. It is a consequence of lipid hydrolysis that is probably mediated by lipolytic acyl hydrolase (Rawyler et al., 1999). When membrane integrity is lost, the cell is irrecoverably damaged (Zhang et al. 1992). Unlike in some animal tissues, there is little evidence that ion channels and carrier systems can become sealed in response to anoxia thereby helping to retain soluble cell contents.
The modest ATP generation capability of glycolysis/fermentation depends on a ready supply of glucose and its precursors. Sugar shortage caused by anaerobic arrest of starch breakdown and sugar unloading in roots can thus shorten the duration of survival. This is illustrated by the ability of rice seeds to germinate without oxygen. Such ability is due, in part, to its possession of an anaerobically inducible gene coding for α-amylase, the enzyme principally responsible for degrading starch to a range of sugars (Loreti et al. 2003). In in vitro studies of anaerobic roots, hexose feeding is a prerequisite for long periods of anaerobic survival of the cultures. Over shorter time scales too (hours or several days), seedling roots have also been found to survive longer and ferment more vigorously when given external glucose (Webb and Armstrong, 1983), Tadege et al., 1998). But, even when anoxic roots are given extra hexose they die eventually, indicating causes of death other than simply substrate-starved arrest of glycolysis. This may be because rates of glycolysis cannot speed-up sufficiently to satisfy demand or because ATP demand is not sufficiently down-regulated. But, other factors also come into play. These include the absence of molecular oxygen to support essential non-respiratory oxidative and oxygenation reactions (e.g., synthesis of polyunsaturated fatty acids used in membrane formation (Vartapetian, Mazliak, and Lance, 1978).
Self-poisoning
Anaerobic roots may also die from self-poisoning by products of anaerobic metabolism; the most notable toxin being excess protons that acidify the cytoplasm and vacuole (Gerendás and Ratcliffe, 2002). In support of this notion, roots of pea (Pisum sativum), black eyed peas and navy beans, which collapse particularly quickly when anoxic, acidify their cytoplasm more rapidly than do longer-lived anoxic maize, soybean or pumpkin root tips. The sources of the extra protons within the cell have proved difficult to identify (Gerendás and Ratcliffe, 2002). Another possible toxin is acetaldehyde. In alcoholic fermentation, activity of the enzyme that converts acetaldehyde to ethanol (alcohol dehydrogenase – ADH) usually exceeds that of the enzyme that promotes acetaldehyde production from pyruvic acid (pyruvate decarboxylase – PDC). Normally, this state of affairs ensures low sub-toxic concentrations of acetaldehyde in anoxic cells. However, after such tissue is returned to air, this control is sometimes lost and plant tissue typically generates a burst of acetaldehyde that could be damaging (Boamfa et al. 2003). Another potential toxin is nitric oxide (Dordas et al. 2003). This is a free radical gas that can be formed by the action of nitrate reductase (coded for by an anerobically inducible gene) and possesses the ability to kill cells, as is well-known in mammalian tissues. However, the roles of this molecule are still very unclear and it has even been suggested that the beneficial impact of nitrate on survival of anoxia may be an outcome of increases in nitric oxide arising from the reduction of nitrate to nitrite (Stoimenova et al. 2003). The conventional view is that death of the root-tip caused by one or more of the above-mentioned factors threatens the vigour and survival of the entire plant since fully functional root tips are required for soil exploration, uptake of water and mineral nutrients. However, Subiah and Sachs, 2003 take the view that rapid death of anoxic root tips is an adaptive response. They consider that loss of the root tip allows the remainder of the root (with its dormant lateral root primordia) to survive for longer, an interpretation supported by their finding that prior removal of root tips prolongs the life of anaerobic maize seedlings.
Although not strictly self-poisoning, cell death arising from oxidative reactions following the re-introduction of oxygen cannot be excluded as a cause of waterlogging injury and death. Underlying this notion is that an absence of oxygen harms the ability of plant cells to protect themselves against the formation and action of active oxygen species (e.g. superoxide radicals) when the floodwater recedes and free oxygen returns to the cells. Roots of soybean are thought to suffer in this way (Van Toai and Bolles, 1991). Most information on this phenomenon comes from work with rhizomes and cultured cells. However, recent studies with cell cultures do not strongly support to the view that post-anoxic damage is a major cause of death from anoxia (Rawyler et al. 2003).
How roots survive anaerobic conditions
During natural waterlogging of the soil, anoxia will be preceded by a period of partial oxygen shortage (hypoxia). This will last for as long as it takes for dissolved oxygen in the floodwater to be consumed by roots and other aerobic soil organisms. This hypoxic interlude can act as a training period by improving the ability of the roots to survive subsequent anoxia by inducing biochemical acclimation or anatomical acclimation.
Biochemical acclimation
As little as 6 h prior exposure to partial oxygen shortage (typically 3 – 5 %, v/v in the gas phase) can lengthen survival time of anoxic maize root tips from 8 h to 72 h (Saglio, Drew, and Pradet, 1988). The mechanism by which cells initially sense the partial oxygen shortage that triggers this acclimation is unknown. One possibility is that sensing works through a binding of oxygen to non-leguminous haemoglobin, which is ubiquitous in plants. However, this mechanism has been ruled out on the grounds that binding is too tight for sensitive detection of partial oxygen shortage (Dordas et al. 2003). Despite this, haemoglobin is undoubtedly important for anoxia tolerance in other as yet undiscovered ways (Hunt et al. 2002) and petunia plants transformed with a Vitreoscilla haemoglobin gene, show remarkably enhanced tolerance to submergence (Imao et al., 2003).
Following sensing of partial oxygen deficiency, genes coding for so-called anaerobic proteins (actually hypoxically-induced proteins or HIPs) are up-regulated at transcriptional and post- translational levels while others coding for many aerobic proteins remain expressed and translated up to the point when the cells finally become anoxic. (Chang et al. 2000; Dolferus et al. 2003; Fennoy and Bailey-Serres, 1995; Subiah and Sachs, 2003; Baxter-Burrell et al. 2003). The anaerobic proteins (or HIPs) are necessary for the acclimation. The up-regulation of these genes is effected by the action of proteinaceous transcription factors (e.g., Myb factors, G-box factors, 14-3-3 proteins) that bind to promoter regions of target genes and influence their expression. The base sequences of these regions determine the susceptibility of a gene to any given transcription factor. One such region is the so-called ‘anaerobic response element’ characterized by a GT/GC-rich motif. This has been associated with several genes, such as an alcohol dehydrogenase (ADH1), that are strongly upregulated by oxygen shortage (Dolferus et al., 1994). HIPs can be divided into (1) enzymes involved in cytosolic energy metabolism especially those involved in starch breakdown and the glycolytic and fermentative pathways upon which anoxic energy generation depends; (2) enzymes implicated in pH regulation; (3) enzymes involved in aerenchyma formation (see later in this article); (4) enzymes with protective functions such as scavenging for potentially damaging active oxygen species generated when anoxic roots are returned to air; (5) proteins involved in signal sensing and transduction (e.g., the ethylene receptor ETR), (6) others of unidentified function. The complexity of the picture is being increased as sophisticated methods of protein analysis such as MALDI-DE-TOF mass spectrometry (Chang et al. 2000) become employed. Thus, HIPs play a major part in prolonging the life of anoxic roots that previously experience at least some hours of partial oxygen deficiency. If their synthesis is interfered with by applying protein synthesis inhibitors or through mutations, the effect of partial oxygen shortage on prolonging survival of subsequent anoxia is suppressed (reviewed by (Jackson and Ricard, 2003). It is notable that translation mRNA of many ‘aerobic’ genes is suppressed in anoxic cells.
Expression of HIP genes and production of survival protein is complimented by a co-ordinated down-regulation of demand both for oxygen, respirable substrates and for ATP. This suppression of demand is instigated by modest decreases in oxygen supply and well in advance of the onset of fermentation or any increase in NADH relative to oxidized NAD+ (Geigenberger et al., 2000). Thus, while HIP production can be seen as a preparation for anoxia, the early down regulation of ATP and oxygen demand appears to delay the point at which tissue anoxia actually sets in. This down regulation of ATP- and oxygen-consuming pathways is probably underpinned by changes in gene expression. As previously mentioned, roots that are partially deprived of oxygen (typically 5 %) undergo marked changes in expression (Andrews et al. 1994; Klok et al. 2002). The kinetics of the up- and down-regulation of various groups of genes is complex with transcription factors and signal transduction pathway genes changing soonest. The identification of gene clusters bearing common regulatory sequences is a priority since by this means the overall co-ordination of events will become clearer (Dolferus et al. 2003). It will be important to understand both the down-regulation of genes coding for energy-consuming steps and the up-regulation of genes coding for survival proteins. Post-transcriptional processing of survival proteins and their patterns of association will be a further priority
Anatomical acclimation through aerenchyma formation
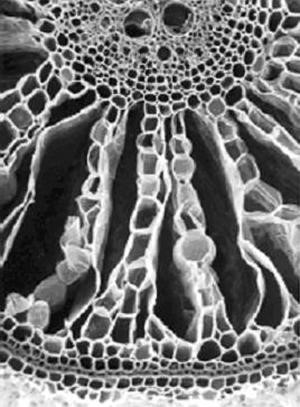
The presence of large interconnected intercellular gas-filled spaces that often extend from the shoots to near the root tip (aerenchyma) is feature shared by most (Justin and Armstrong, 1987b) although not all species (e.g. Caltha palustris – Seago et al. 2000) that grow well in wet places. The spaces are created by cell separations resulting from differential rates of division or expansion by neighbouring cells or from the death of certain cells. The radially orientated spaces of the rice root is an example of aerenchyma formed by the death of particular cells that takes place mostly as a part of normal constitutive development (Fig. 4). Mathematical modelling and direct experimentation have demonstrated that sufficient oxygen can diffuse through such tissue from the aerial shoot to satisfy the respiratory needs of root axes up to 30-cm-long at growing temperatures. In certain aquatic species, pressure-driven gas flow may aerate even longer lengths of rhizomes to which roots are attached (reviewed in (Jackson and Armstrong, 1999). Tests with wheat plants have shown that, without aerenchyma, roots longer than 100 mm are fatally damaged by an O2-free medium while roots of this length equipped with 12 % porosity continue elongating. The effectiveness of oxygen transport is increased in species like rice where oxygen losses to the soil are inhibited by an inducible barrier to outward radial diffusion (Colmer et al., 1998). Any oxygen leaking radially out of the roots into the anaerobic soil can oxidise the rhizosphere thus decreasing injury from chemically reduced toxins such as ferrous ions.
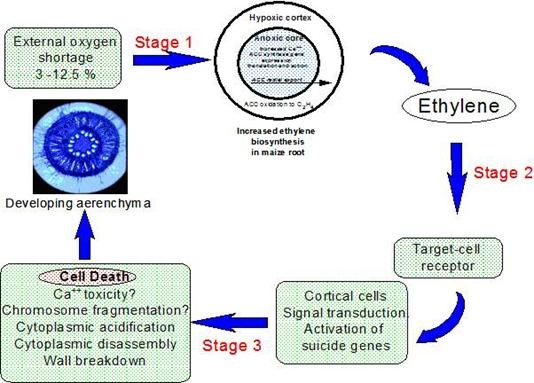
However, most species are intolerant of soil waterlogging and do not possess extensive aerenchyma (e.g., arabidopsis and tomato) but, in some (e.g. Zea mays), aerenchyma in roots can be stimulated during the early stages of waterlogging by the associated partial shortage of oxygen. This morphological acclimation is the result of spatially targetted process of programmed cell death that is localized in the root cortex and is stimulated by increases in ethylene concentration (Drew et al. 1979) brought about by entrapment and by faster biosynthesis; the latter being favoured by low but not extinguished oxygen supply e.g. 0.68 mol m-3 or 5 % v/v oxygen) (Fig. 5). The additional ethylene is trapped within the flooded roots by the floodwater and acts on files of target cells in the cortex resulting in their disassembly to create longitudinally interconnected gas spaces. Processes of cellular disassembly that destroy the cell are only partially understood and the state of knowledge is summarised in Fig. 5. This so-called lysigenous aerenchyma is the outcome of an ordered set of structural degradative changes (lysis) that commence within 6 h and destroy the cell in 2-3 d. These changes include, acidification of the cytoplasm, possible loss of control of cytoplasmic Ca++, a loss of microtubule orientation, plasmamembrane invagination, the formation of membrane-bound vesicles and, the appearance of membrane bound organelles, internucleosomal DNA fragmentation, chromatin condensation and changed patterns of pectin methylation in cell walls (Gunawardena et al. 2001a) and (Gunawardena et al., 2001). The features of those cortical cells that confer target status for ethylene induction of programmed cell death remain elusive.
How above-ground shoots survive soil waterlogging and submergence
It is inevitable that, because of the close functional interdependence between roots and shoots, stress on roots from soil waterlogging also threatens the shoot system. One example of this is the arrest of nitrate uptake that arises from microbial de-nitrification and damage to uptake mechanisms from an absence of oxygen. Young leaves then take this nutrient from older leaves leading to premature senescence in the latter (Drew and Sisworo, 1977). A second example of this knock-on effect on shoots is the tendency of waterlogged plants to wilt severely in bright light. This paradoxical response to waterlogging of the soil is the outcome of a lowered conductivity to water uptake that typifies oxygen-deficient roots and is brought about by proton induced conformational changes to water channel proteins (Tournaire-Roux et al. 2003).
Root to shoot communication and shoot-water conservation
The tendency for leaves to dehydrate irreversibly (Fig. 6) in response to increases in root hydraulic conductivity is ameliorated by rapid signalling from roots to shoots that results in a slowing of water loss from the foliage. This is achieved by a prompt decrease of stomatal apertures, leaf expansion and, in tomato at least, by marked petiolar epinasty. Epinasty is the outcome of a downward re-orientation of whole leaves and leaflets that involves growth promotion on the upper (i.e., adaxial) surface. Epinasty reduces the amount of energy incident on the foliage and thus will slow the rate of water loss.
The signals inducing these effects in the shoots have been reviewed recently (Jackson, 2002) and, in the main, comprise increases (positive messages) or decreases (negative messages) in the flux of regulating hormones, or related substances, carried to the shoot in xylem sap. The signalling processes controlling epinasty in tomato are well understood, involve ethylene and can be summarised as follows. Severely hypoxic or anaerobic roots were shown to generate a positive message that (Bradford and Yang, 1980) showed later to be the ethylene precursor 1-aminocyclopropane-1-carboxylic acid (ACC). The amounts have been estimated using mass spectrometry and are demonstrably sufficient to enrich the shoot tissues with enough ACC to support the faster shoot ethylene production needed to induce epinasty (Else and Jackson 1998). The release of large amounts of ACC into xylem sap by anaerobic roots has two probable causes. The first is a blocking of ACC oxidation to ethylene by the absence of oxygen. This promotes a build-up of ACC. Some of this enters the transpiration stream and is drawn in to the shoot by transpiration steam as positive message. This enrichment has been shown in tomato and in xylem sap of R. palustris. The second putative cause is an up-regulation, and presumed translation of one member (LE-ACS7) of the six-gene family of ACC synthases responsible for forming ACC from its precursor S-adenosyl-L-methionine. Up-regulation occurs within 1 h and takes place along with a rise in ACC concentration in roots and enhanced ethylene production in the leaves (Shiu et al. 1998). Changes in gene expression and enzyme levels also enhance the oxidation of ACC on its arrival in the shoot since the ability of petioles to oxidase ACC to ethylene increases within 6 h (English et al. 1995) along with a rise in ACC oxidase mRNA. However, it remains unfortunate that detailed time courses of ethylene production using modern sensors such as photoacoustics, are not available to confirm the link between the onset of epinasty and increased shoot ethylene production.

Fast stomatal closure is often seen in many species soon after waterlogging begins but has proved difficult to explain. In tomato, many potential signalling messages such as increases in delivery of the hormone abscisic acid (ABA), sharp decreases in mineral nutrient delivery or increased xylem sap alkalinity have received little support, although in Ricinus communis, temporary dehydration of the leaves induced by severely decreased root hydraulic conductivity appears to trigger stomatal closure that is mediated by ABA (Jackson et al., 2003). Other less well-researched possibilities for root to shoot signalling in waterlogged plants involve decreases in pH and in the delivery of cytokinin or gibberellin hormones. In addition to possibly contributing to closing the stomata these changes may also help explain other shoot responses to soil waterlogging such as depressed stem elongation rates and faster leaf senescence.
Root to shoot signalling is best seen as a stop-gap mechanism that rapidly reduces the need for root-sourced supplies such as water and minerals at a time when root activities are suppressed by inadequate oxygen. But, if the roots remain inactive, or are killed and not succeeded by functional replacements, the shoot will not survive and the plant will then fail in its entirety. For longer-term survival of the plant, oxygen must be introduced into the interior of roots in amounts that support respiration and activities such as mineral and water uptake. Alternatively, replacement roots must develop that are better aerated than their predecessors. In species with adaptive capacity, shoots respond to flooding in ways that favour such changes. These are discussed briefly below.
Increased porosity of the shoot base
In many species the shoot base become morphogenetically activated when plants are waterlogged, especially if the shoot base itself becomes water- covered. In Z. mays, the base of outer leaves form aerenchyma (Fig 7A) and studies in rice reveal a link with transverse veins and a distinct interconnectedness between the cells that eventually die to create the aerenchyma (Matsukura et al. 2000). In many dicots, swelling of submerged portions of the lower shoot and the development of swollen stems and hypertrophied lenticels are common. These effects probably enhance flooding tolerance by promoting tissue gas exchange, although experimental data demonstrating this are elusive. Swelling and hypertrophic lenticels are seen in many herbaceous dicots and woody species (e.g., apple – Fig. 7B) (Hook and Brown, 1973). Experimental blocking hypertrophic lenticels with lanolin grease has been shown to decrease oxygen entry into nearby roots via intercellular spaces and aerenchyma. This oxygen is then potentially available to support root functioning and also the formation and emergence of new adventitious roots nearby (see next section). Hypertrophic lenticels may also allow dissipation of metabolically generated volatiles such as ethanol, ethylene and acetaldehyde, although the physiological significance of this for plant performance and survival has not been examined. The leaf-based aerenchyma, and swelling of stem-base and lenticels are probably consequences of cell expansion promoted by endogenous ethylene trapped in the submersed tissue by the water covering (Kawase, 1974).
Replacement rooting
Species with inherently surface-inhabiting root systems are notably tolerant of prolonged waterlogging (Justin and Armstrong, 1987a). However, if those species with deeper and thus more vulnerable roots are to revive, they must form replacement roots positioned near or at the better-aerated soil surface. There are three mechanisms for generating these replacement root systems. One is a stimulation of the outgrowth of root primordia already present within the shoot base (e.g., in Zea mays – Fig.8A). A second is the induction of a new root system that involves initiation of root primordia and their subsequent outgrowth (e.g. in Helianthus annuus [Fig 8B] and Rumex palustris). Ethylene seems to be involved in both these processes. Applications of the gas to Z. mays strongly promotes primordial emergence (Jackson et al. 1981) although subsequent elongation is inhibited in association with aerenchyma formation. In nature, this inhibition may be mitigated by internal ventilation of ethylene out through the aerenchyma (Visser et al. 1997). Where replacement rooting involves initiation of new root primordia, auxin and ethylene are both thought to interact to bring this about (Visser et al., 1996). A third mechanism of placing roots at the soil surface involves a re-orientation of the root extension. Lateral roots of certain species grow upwards in waterlogged soils (Pereira and Kozlowski, 1977); Gibberd et al. 2001). When they reach the surface, they offer a replacement pathway for aeration of other attached roots provided there is adequate internally interconnected aerenchyma.
![Fig. 8. Effect of approximately two weeks waterlogging on the formation of replacement adventitious roots at the soil surface by plants of [A] maize (Zea mays) and [B] sunflower (Helianthus annuus). In A, the roots are outgrowths of pre-existing primordia. In B, the original root system has been killed and replaced by newly initiated roots formed at the shoot base and hypocotyl. Both mechanisms generate a root system with improved access to aerial oxygen](https://plantstress.com/wp-content/uploads/2020/06/water9.jpg)
Fast upward shoot elongation
Another developmental effect of flooding, that supplements the aerenchyma system, is the promotion of shoot extension and/or increased uprightness of submerged leaves (Grimoldi et al. 1999; Cox et al. 2003). These responses are mostly found in aquatic or amphibious species well-adapted to periodically flooded areas or to rising water levels (e.g., rice and water lilies). The resulting increase in effective plant height, that can begin after a delay of less than 2 h of inundation, improves access to aerial or dissolved oxygen or to light for the generation of photosynthetic oxygen. In appropriate species, this oxygen can diffuse readily to the root elongation zone and elsewhere through aerenchyma (Waters et al., 1989). Internodes of the highly compressed stem in the base of the shoot of Z. mays are also stimulated to elongate when water levels cover just the shoot base. This raises the height of the ring of replacement roots emerging from a basal node. For the most part, the promotion of elongation by shoot or leaf are primary responses to ethylene entrapped in the growing tissue by the floodwater (Musgrave, Jackson, and Ling, 1972). The hormone is known to act in conjunction with gibberellins and/or with auxin to stimulate cell extension. This effect is in marked contrast to the effect of ethylene on less-well adapted species, where the gas most often strongly inhibits shoot elongation. In stems of deepwater rice (Kende et al., 1998) and petioles of Rumex palustris (Voesenek et al., 2003), faster underwater elongation is strongly dependant on a prior degradation of the growth-inhibiting hormone abscisic acid. Faster extension is also associated with the expression of a gene encoding a member of the family of cell-wall loosening enzymes known as expansins and with the up-regulation of an ethylene receptor gene (Voesenek et al., 2003). The range of species that utilize ethylene in this depth-accommodation response is wide and includes dicots, monocots and at least one liverwort and a tropical fern (Regnellidium diphyllum) (Table 2). However, the mechanism is not universal. In Potamogeton pectinatus, submerging the stem promotes elongation even though the plant is unable to synthesise ethylene itself because of ACC oxidase activity is lacking (Summers et al., 1996). Leaves of rice seedlings may also respond to a signal other than ethylene (Ella et al., 2003). Clearly, some other signal can be generated by submergence that is responsible for stimulating elongation. That signal may comprise accumulated carbon dioxide or partial oxygen shortage since rice coleoptiles elongate faster underwater in response to their collaborative effects as well as to ethylene. In the stems of deepwater rice, these signals act more independently (Kende, 1987). Young rice seedlings are readily submerged in water too deep for them to escape by means of fast upward elongation. In these circumstances, the expenditure of energy imposed by the faster growth rates may prejudice their survival. Survival is also challenged by more extensive leaf senescence that is promoted by accumulated ethylene (Jackson and Ram, 2003; Ella et al. 2003). In lowland rice types, those most tolerant to several days of total submergence as small seedlings (e.g. FR13A) are usually those that elongate very little underwater while retaining a full set of green leaves.
The most severe stress that flooding can impose on the shoot is total immersion in dark, anaerobic surroundings. Green leaves of some species tolerant of total submergence and of icing-over in arctic environment have the ability to survive such conditions, as do perennating organs of many temperate wetland species (Crawford and Brändle,1996; Schlüter and Crawford, 2001). A variety of biochemical adaptations reminiscent of those discussed earlier for roots are involved although studies with the dicot seedlings of Arabidopsis thaliana (Ellis et al. 1999) indicate that the biochemical pathways involved in adaptation to very low oxygen supply (approx. 0.1 %) may be different in roots and shoots. In addition, a select group of species including Potamogeton pectinatus, Potamogeton distinctus, Sagittaria pygmaea and rice can respond to a complete absence of oxygen by elongating their stem (or coleoptile in rice) much more quickly in an upward, gravity sensitive manner for many days. The biochemical basis of this remarkable achievement includes being able to degrade starch anaerobically and to sustain a fast rate of glycolysis in the growing parts (Summers et al. 2000). The faster growth is principally the outcome of enhanced cell extension linked to the promoting action of low pH and to the action of auxin that is implicated in anaerobic gravity sensing (Summers and Jackson, 1996). This stimulation of upward shoot elongation by a complete absence of oxygen is coupled with a marked constitutive aerenchyma. The two features together constitute a mechanism of survival by escape from flooding stress by means of upward elongation. But in this case the signal promoting fast elongation is, surprisingly, the complete absence of oxygen, even when this most severe stress is prolonged for many days
WATERLOGGING AND CROP PRODUCTION
Remedial measures in crop production
o Improving land drainage
o Direct treatment to crops
o Plant breeding
Based on soil typing, (Dudal, 1976) estimated that 12 % of the World’s soils are likely to suffer from excess water while (Boyer, 1982) indicated that about 16 % of US soils experience excess water. Clearly, considerable transient and more persistent waterlogging of the soil and deeper submergence of crops occurs in much rainfed farmland world-wide. The extent of its occurrence remains speculative. This is because of a lack of useful definitions of what constitutes excess water content. The transient nature of most farmland flooding also frustrates accurate estimation of the extent of farmland waterlogging. One potentially useful rule of thumb (discussed in Setter and Waters, 2003) is the SEW30 value. This value (as centimeter days) combines all the days in a growing season when excess water is present in some or all of the top 30 cm of the soil profile (i.e. the zone in which most roots are usually to be found). Values of about 100 – 200 cm d are often sufficient to depress crop growth in temperate growing conditions.
Despite the uncertainties of quantification, it is possible to give examples to indicate the scale of the problem. Through such an approach we can appreciate that although statistically, floods are amongst the most common and widespread of all natural disasters and the effects are sometimes catastrophic (e.g., Mozambique in 2000), it is the more mundane persistent inadequacies in soil drainage in the face of near-average or modestly excessive rainfall that constrain farm productivity year by year (e.g., Fig. 9). North Dakota State University Extension Service reports that throughout the 1990s, excess rain affected every area of the State. The impact of flooding on cropping has been particularly well studied on the duplex soils of Western Australia (McFarlane and Cox, 1992). Each year, wherever rainfall exceeds approximately 400 mm, waterlogging occurs in a minimum of 8 % of the total cropped area i.e., 1.3 million ha of pastures and approx. 500,000 ha of cultivated land. The area of the latter may be increasing as arable farming expands because of poor returns on animal husbandry. For the 250,000 ha of barley grown in Western Australia, waterlogging is thought to reduce yields by 20-25 %. Assuming waterlogging in 2 out of 5 years, the value of the lost yield has been calculated at 6.84 million Australian Dollars each year. In 1988, crop losses due to waterlogging in a 27000 ha catchment area around Yornanning were approximately 1.1 million Australian Dollars from barley alone. In a 700,000 ha sandplain area, approximately 400,000 ha are subject to transient waterlogging, making financially viable lupin production almost impossible. Furthermore, these waterlogged soils are believed to be sites of groundwater recharge, contributing to serious salinization of the sandplain. In the state of Victoria, 1.8 million ha of duplex soils are affected by waterlogging, with another 2.3 million ha are susceptible. This is estimated to cost the state of Victoria 36 million Autralian Dollars in lost crop production each year. Setter and Waters (2003) give other examples such as the wheat-growing regions of northern India.

Waterlogging damage to crops may sometimes occur when crops are irrigated. The dividing line between adequate and excessive irrigation is not well defined and it is probable that some of the benefits can be lost by heavy-handed irrigation. For example, ponding on the soil surface for more than a day is known to be harmful to wheat watered by flood-irrigation. Prolonged irrigation over many years in dry regions can cause waterlogging problems created by raising the water table. A well-known example, already mentioned, is to be found in the Sindh Province in the Indus Valley of Pakistan. Here, 50 to 60 years ago, the watertable was 4 m below ground. By 1980s it had risen to between 1 – 2 m over most of the irrigated region and the water being highly saline. A multimillion-dollar drainage project (the Left Bank Outfall Drainage project) intended to reverse the situation and funded by the World Bank and the Asian Development Bank is in place but not without causing controversy and additional environmental concerns.
The extent of damage to yield depends heavily on the stage of development as well as on more obvious factors such duration of waterlogging and temperature. For most crops, seed germination is probably the most vulnerable, reflecting both the fast metabolic rate of germinating seeds being coupled with complete inundation. Studied examples include peas (Jackson, 1983) and temperate cereals (Lynch et al., 1981), (Setter and Waters, 2003). Problems are exacerbated if germination takes place in association with decomposing organic matter such as straw residues from the previous crop. Microbial colonization of such residues depletes the soil of oxygen and nitrate while discharging potentially harmful substances such as unsaturated fatty acids and the growth-inhibiting hormone abscisic acid. Leaking of soluble carbohydrates from seeds is stimulated by anaerobiosis and this also encourages fungal pathogens such as Gliocladium roseum. Seeds of rice are exceptional in being able to germinate in flooded soil without oxygen. But the germination is abnormal since only the coleoptile but not the root or the mesocotyl or leaves emerge from the embryo without oxygen. Elongation by the emerging coleoptile is stimulated under these conditions by a combination of lack of oxygen, carbon dioxide and ethylene. Underpinning this is the ability of rice seeds to hydrolyse endosperm starch to sugars by means of anaerobically transcribing genes, such as the Ramy3D α-amylase gene, that are up regulated by the associated low sugar levels (Loretti et al. 2003).
Once germinated, stages in subsequent development of crop plants influence susceptibility to flooding injury. Small cereal seedlings with their shoots below ground are highly susceptible (e.g., winter wheat – (Cannell et al. 1980) but thereafter tolerance rises until early reproductive stages when susceptibility again increases (e.g. in soybeans – Linkemer et al. 1998). Heightened vulnerability at or just before flowering has also been noted for several other crops including peas, wheat, sorghum, maize and cow peas (Cannell and Jackson, 1981) when inundated for one or more days. By contrast, several weeks of waterlogging in winter of young plants of crops such as autumn sown wheat or oil seed rape causes little yield loss because of compensatory growth in the following spring and the slower metabolic demands created by cool temperatures during the period of waterlogging.
Unlike other major crop species, rice can yield heavily when grown in waterlogged soil (paddy rice production) and specialized ecotypes are able to yield usefully even when partially submerged in several meters of water (Catling, 1992). This ability is based partly on ethylene-mediated stem elongation that is induced when the shoot base becomes submerged. However, it is less widely recognized that these do not readily escape total submergence. Small vegetative rice plants (even of the so-called deepwater ecotypes) are totally submerged for only a few days as a result of uncontrolled flooding in lowland areas, they are severely damaged or killed. Part of the problem is thought to be that stimulated leaf elongation and associated ethylene-promoted leaf senescence quickly deprive the young plants of starch and sugars, thus prejudicing their survival and re-growth potential (Jackson and Ram, 2003).
Remedial measures in crop production
A comprehensive treatment of remedial measures is outside the scope of this review. However, a brief account of various approaches is given. It pre-supposes that, where appropriate, measures such as installing protective barriers against water inflow from rivers or the sea are installed in association with high-volume pumping systems. The creation and maintenance, by these means, of highly productive farmland in reclaimed coastal areas of The Netherlands represents the pinnacle of achievement in this regard. A common feature in the management of this and other riverine farmland (e.g. The Mississippi floodplain) is the building of dykes or levees (long high banks) along rivers to prevent them from overflowing or at least displacing the flooding to lower reaches of the river. The approach has its dangers.
Improving land drainage
Estimates of the areas of drained farmland that have been published for several major arable-cropped regions. While highly approximate, they serve to illustrate the enormous scale of the problem of farmland waterlogging and the key role of drainage systems in supporting economically viable agriculture. Nosenko and Zonn (1976 – quoted by Cannell and Jackson 1981) estimated that 155 million ha carried subsurface land drains. Much draining has been installed in the last 50 years (e.g. 6 % of UK farmland received new drains between 1940 and 1970). Most of these systems have been installed to arbitrary drainage targets, reflecting the scarcity of drainage experiments on representative soils.
According to Trafford (1975), installation of drainage systems may be motivated by four rather different sets of circumstances. (1) Where reclamation of flooded land is needed before conventional agriculture is possible (see above); (2) where farming of a given crop may have to be abandoned unless drainage is installed (e.g., white lupin production on the south coast of Western Australia); (3) where improved drainage is needed to support more intensive and higher-value cropping and (4) drainage to improve the yields and profitability of an existing cropping system. In situations (3) and (4) the availability of subsidies to fund part of the cost of installing land drains may affect the financial viability of installing land drains. On a clay soil in the UK, even a 28 % improvement in yields of winter wheat reported over a six-year period after installing pipe and mole drains would not have been an economic investment unless installation was subsidized by government grants (Hunter and Trafford, 1979). Such subsidies are no longer available in the UK and much of western Europe. However, less financially accountable benefits such as a marked increase in the number of days the drained land can bear heavy farm machinery without damage must not be ignored.
Mention has already been made of Pakistan’s Left Bank Outfall Drain (LBOD) project, one of world’s largest drainage schemes. It highlights the installation of land drainage schemes as one of the most widely adopted means of decreasing the incidence of farmland waterlogging. At the individual farm level, measures for improving drainage range from simple and historically interesting ridge and furrow constructions (basically creating raised beds for the crop – system sometimes used in Australia) to the installation of complex subsurface land drains designed to meet target drainage flows and installed using laser-guided drain layers that insert perforated plastic piping to precise depths and gradients. The use of such systems is illustrated by guidance given by the USDA-Agricultural Research Service to sugarcane growers in Lower Mississippi River Valley (LMRV). The machinery used in such work can be formidable (Fig. 10). In contrast the simplest subsurface land drains are those created in clayey soils using a ‘mole’ that is dragged through the soil at a depth of about 50 cm. Despite its seeming simplicity, powerful tractors are still required for its execution (Fig. 11). A combination of the two forms of drainage installation is especially effective. Other means of lowering the water table include the judicious planting of waterlogging tolerant trees such as certain eucalyptus species, guava and mango that also produce a useful crop for cash-poor farmers in poorly developed tropical regions. Eucalyptus aggregata, E. camphora, E. crenulata, E. gunnii & E. gunnii divaricata are especially tolerant of waterlogging. In New Zealand and Tasmania, they grow naturally in undrained peat moors where surface water is present for at least six months of the year. They also tolerate stagnant water just below the surface (unlike poplar and willow). They are, however, stunted on permanently flooded soils. Another approach aimed at reducing the rate of water input into the lower reaches of floodplains such that of the River Rhine in northern Europe, is to retain as much water as possible upstream. This can be improved by preserving and improving what remains of upstream flood plains (so-called soft engineering).

Direct treatment to crops
Remedial effects can be observed after applying nitrogen fertilizers as a foliar spray. There is evidence of beneficial effects of this on the yield of cotton http://cotton.crc.org.au/Publicat/Agro/waterlog.htm. Cereals may also benefit from soil applications of N provided the plants are only moderately damaged. In Western Australia, 100kg N ha-1 or more raised yields of small grain cereals waterlogged for 3-7days but little benefit is seen in more severely damaged crops. The use of additional nitrogen to offset waterlogging damage has support from basic physiological investigations. These show that applied nitrate may enter anaerobically damaged roots by passive means and be translocated to the shoot. The nitrate may also act simply to replace that leached in the drainage water or destroyed by anaerobic denitrifying bacteria (Trought and Drew, 1981).
Germinating seeds have been identified as being especially vulnerable to damage from soil waterlogging and the associated anaerobiosis and injury from chemically reduced metabolites such as organic acids and respiratory intermediates such as acetaldehyde. It is therefore not surprising that pre-treating seeds with an oxidizing coat has been tried as an insurance against such effects. Coatings include calcium hydroxide and calcium peroxide (Sladdin and Lynch, 1983), although the effects on rice have not always been positive. There are companies selling products that promise to improve plant growth by using calcium peroxide to ‘inject’ oxygen in to the soil. A more mundane approach in situations where germinating seeds risk contact with undegraded crop residues in wet conditions, is to rotovate and dice these residues or remove them before sowing, possibly by burning if legislation allows this.
Plant breeding
As with other major abiotic stresses, breeding and selecting successful tolerant cultivars have not yet met with notable commercial success although Setter and Waters (2003) report that a waterlogging tolerant wheat cultivar suitable for Australian conditions is close to being released. This has been derived from doubled haploid (DH) lines generated by crosses with tolerant wheat originally selected by the International Maize and Wheat Improvement Center (CIMMYT) in Mexico. Despite this limited progress, there are numerous indications that the production of cultivars with improved tolerance that also retain their desirable agricultural traits is a realistic prospect for several major food crops. The utility value of much published work depends on the criteria used to assess tolerance. Not all reports compare final yields, or include non-waterlogged controls in their trials. Nevertheless, a basis for future successful breeding can be found in reports of greater than normal tolerance in a number of major crop species, or allied species that could perhaps be employed to introduce tolerance traits through ‘wide hybridization’. For example, waterlogging-tolerant accessions have identified in seven of the eight taxonomic sections in Trifolium when compared in terms of relative growth rates. Several lines of soybean and of winter wheat have also been shown to possess unusually high tolerance on the basis of injury levels and final yield. Comprehensive tests in the glasshouse and in the field over several seasons and involving yield comparisons of over 20 cultivars (Musgrave and Ding, 1998) revealed notable tolerance in two lines of winter wheat that was positively correlated with iron-rich surface deposits on the roots, implying a link with increased amounts of aerenchyma. Based on levels of chlorosis in waterlogged spring wheat, (Boru et al. 2001) concluded that tolerance was largely controlled by four genes with beneficial effects that could be additive. (Setter and Waters, 2003) have assessed, in detail, many other reports of inheritable variation amongst the major small-grained cereals. Examples of variability in tolerance in a variety of other crops are given by (Cannell and Jackson, 1981).
Linking tolerance with identifiable phenotypic traits, such as aerenchyma may help guide evaluation and selection processes associated with breeding. For example, Garthwaite et al. (2003) linked tolerance in certain wild accessions of Hordeum and a modern barley cultivar with both constitutive aerenchyma in the roots and with the development of an effective barrier to radial oxygen loss that renders internal oxygen transport towards root tips more efficient. Interestingly, this barrier developed most strongly in accessions collected from naturally wet habitats. However, in a recent survey of spring wheat lines (Boru et al. 2003) could not link aerenchyma and tolerance. Instead more tolerant types adopted abnormally fast aerobic respiration rates as external oxygen supplies decreased. Despite this finding, the possession of a highly porous aerenchyma system in association with slow radial losses remains a high priority marker for waterlogging tolerance that may also be introduced through wide hybridization (Comis, 1997).
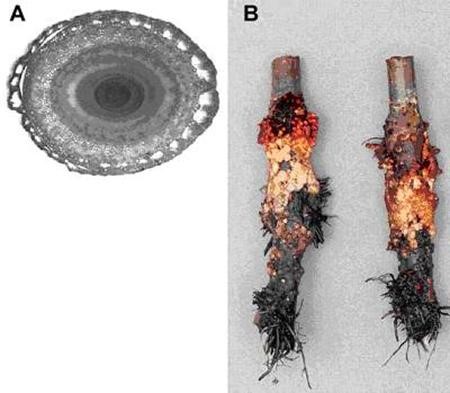
Breeding flood-tolerant crops would be assisted by the development of reliable genomic markers that are linked closely to key tolerance traits. A prerequisite is the existence of inheritable tolerance and Quantitative Trait Locus (QTL) mapping of the traits on to a chromosome linkage map. This is being attempted in soybean using homozygous Random Inbred Lines (RILs) derived from crosses between tolerant lines obtained from China and hybridized to standard North American cultivars. In lowland rice, notably high tolerance to complete submergence displayed by an old farmer variety from northern India (Fig. 12) has been used to create populations of doubled haploid and random inbred lines by crossing with intolerant types. Analysis of the progeny has revealed one particularly strong QTL for submergence tolerance on chromosome 9. Sequences close to this QTL have clear potential for use as tolerance markers in breeding programmes (e.g. Xu et al. 2004). Recent breeding and selection work has shown that when a tolerant line was crossed with an agronomically important but submergence intolerant one (a Thai fragrant rice), both tolerance and the marker were successfully transferred and remained linked even after five backcrossings (Siangliw et al. 2003). Molecular markers for submergence tolerance in rice are becoming even more dependable as fine mapping identifies sequences that are ever closer to the actual gene(s) responsible for conferring tolerance (Toojnda et al. 2003).

A more long-term approach, requiring prior knowledge of key traits or pathways affecting tolerance, is genetic transformation. The limited progress so far is not without promise. (Quimio et al. 2000) reported improvements to submergence tolerance in rice by transforming rice with a gene (rice pdc1) coding for pyruvate decarboxylase the presumed rate-limiting step in ethanolic fermentation. A similar result has been obtained with arabidopsis but not in tobacco. According to Zhang et al. (2000), tolerance to submergence or to waterlogging of the soil can also be improved by transforming arabidopsis with a bacterial isopentenyl transferase gene that enhances cytokinin hormone production when associate with a senescence promoter sequence. Petunia and arabidopsis transformed to over express a haemoglobin gene have also shown remarkable resilience to submergence stress (Imao et al., 2003) or severe hypoxia (Hunt et al. 2002). Neither species can be considered a major crop but similar transformations using important food crop species are anticipated.
ACKNOWLEDGEMENTS
I thank Dr T.D. Colmer (university of Western Australia) and Professor L.A.C.J. Voesenek (University of Utrecht) for helpful comments on an earlier draft of this review.
REFERENCES
This lists references used in the text that are not hyperlinked to internet sources
Andrews, C. J. 1996. How Do Plants Survive Ice? Annals of Botany 78: 529-536.
Andrews, D. L., D. M. MacAlpine, J. R. Johnson, P. M. Kelley, B. G. Cobb, AND M. C. Drew. 1994. Differential Induction of mRNAs for the Glycolytic and Ethanolic Fermentative Pathways by Hypoxia and Anoxia in Maize Seedlings. Plant Physiology. 106: 1575-1582.
Arshad, M., AND W. T. J. Frankenberger. 1990. Production and stability of ethylene in soil. Biology and Fertility of Soils 10: 29-34.
Boru, G., M. van Ginkel, W. E. Kronstad, AND L. Boersma. 2001. Expression and inheritance of tolerance to waterlogging stress in wheat. Euphytica 117: 91-98.
Boru, G., M. van Ginkel, R. M. Trethowan, L. Boersma, AND W. E. Kronstad. 2003. Oxygen use from solution by wheat genotypes differing in tolerance to waterlogging. Euphytica 132: 151-158.
Boyer, J. S. 1982. Plant productivity and the environment. Science 218: 443–448.
Bradford, K. J., AND S. F. Yang. 1980. Xylem transport of 1-aminocyclopropane-1-carboxylic acid, an ethylene precursor, in waterlogged plants,. Plant Physiology 65: 322-326.
Cannell, R. Q., AND M. B. Jackson. 1981. Alleviating aeration stresses. In H. M. Taylor [ed.], Modifying the root environment to reduce crop stress, 141-192. American Society of Agricultural Engineers, St Joseph.
Cannell, R. Q., R. K. Belford, K. Gales, C. W. Dennis, AND R. D. Prew. 1980. Effects of waterlogging at different stages of development on the growth of winter wheat. Journal of the Science of Food and Agriculture 31: 117-132.
Catling, D. 1992. Rice in deep water. The Macmillan Press and International Rice Research Institute, London and Manila.
Comis, D. 1997. Aerenchyma. Lifelines for living underwater. Agricultural Research 45: 4-8.
Cook, C. D. K. 1999. The number and kinds of embryo-bearing plants which have become aquatic: a survey. Perspectives in Plant Ecology, Evolution and Systematics 2/1: 79-102.
Corner, E. J. H. 1964. The life of plants. Weidenfield and Nicolson, London.
Davison, E. M. 1997. Are jarrah (Eucalyptus marginata) trees killed by Phytophthora cinnamomi or waterlogging? Australian Forestry 60: 116-124.
Drew, M. C., M. B. Jackson, AND S. Giffard. 1979. Ethylene promoted adventitious rooting and development of cortical air apaces (aerenchyma) may be adaptive responses to flooding in Zea mays L. Planta 147: 83-88.
Dudal, R. 1976. Inventory of the major soils of the World with special reference to mineral stress hazards., Plant adaptation to mineral stress in problem soils, 3-13. Cornell University, Ithaca, New York.
Geigenberger, P., A. R. Fernie, Y. Gibon, M. Christ, AND M. Stitt. 2000. Metabolic activity decreases as an adaptive response to low internal oxygen in growing potato tubers. Biological Chemistry 381: 723-740.
Gerendás, J., AND R. G. Ratcliffe. 2002. Root pH control. In Y. Waisel, A. Eshel, and U. Kafkafi [eds.], Plant Roots the Hidden Half, 553-570. Marcel Deker Inc, New York.
Greenland, D. J. 1977. Soil damage by intensive arable cultivation: temporary or permanent? Philosophical Transactions of the Royal Society of London (B) 281: 193-208.
Gunawardena, A. H. L. A. N., D. M. E. Pearce, M. B. Jackson, C. R. Hawes, AND D. E. Evans. 2001. Characterization of programmed cell death during aerenchyma formation induced by ethylene or hypoxia in roots of maize (Zea mays L.). Planta 212: 205-214.
Hook, D. D., AND C. L. Brown. 1973. Root adaptations and relative flood
tolerance of five hardwood species. Forest Science 19: 225-229.
Hunter, J. M., AND B. D. Trafford. 1979. An economic appraisal of six years’ results from a drainage experiment on clayland. Experimental Husbandry 35: 1-6.
Imao, Z. C., Y. L. Hu, J. Zhong, L. X. Wang, J. Y. Guo, AND Z. P. Lin. 2003. Improvement of the hydroponic growth and waterlogging tolerance of petunias by the introduction of vhb gene. Acta Botanica Sinica 45: 205-210.
Jackson, M. B. 1983. Plant and crop responses to waterlogging. Aspects of Applied Biology 4: 99-116.
Jackson, M. B., AND W. Armstrong. 1999. Formation of aerenchyma and the process of plant ventilation in relation to soil flooding and submergence. Plant Biology 1: 274-287.
Jackson, M. B., AND P. C. Ram. 2003. Physiological and Molecular Basis of Susceptibility and Tolerance of Rice Plants to Complete Submergence. Annals of Botany 91: 227-241.
Jackson, M. B., AND B. Ricard. 2003. Physiology, biochemistry and molecular biology of plant root systems subjected to flooding of the soil. In H. de Kroon and E. J. W. Visser [eds.], Root ecology. Springer-Verlag, Berlin, Heidelberg.
Jackson, M. B., M. C. Drew, AND S. C. Giffard. 1981. Effects of applying ethylene to the root system of Zea mays L. on growth and nutrient concentration in relation to flooding. Physiologia Plantarum 52: 23-28.
Jackson, M. B., L. R. Saker, C. M. Crisp, M. A. Else, AND F. Janowiak. 2003. Ionic and pH signalling from roots to shoots of flooded tomato plants in relation to stomatal closure. Plant and Soil 253: 103-113.
Justin, S. H. F. W., AND W. Armstrong. 1987a. Anatomical characteristics of roots and plant response to soil flooding. New Phytologist 105: 465-495.
______. 1987b. The anatomical characteristics of roots and plant response to soil flooding. New Phytologist 106: 465 – 495.
Kawase, M. 1974. Role of ethylene in induction of flooding damage in sunflower. Physiologia Plantarum 31: 29-38.
Kende, H. 1987. Studies on internodal growth using deep-water rice. In D. J. Cosgrove and D. P. Knievel [eds.], Physiology of cell expansion during growth, 227-238. American Society of Plant Physiologists, Rockville.
Laanbroek, H. J. 1990. Bacterial cycling of minerals that affect plant growth in waterlogged soils: a review. Aquatic Botany 38: 109-125.
Lynch, J. M., F. B. Ellis, S. H. T. Harper, AND C. D.G. 1981. The effect of straw on the establishment and growth of winter cereals. Agriculture and the Environment 5: 321-328.
McFarlane, D. J., AND J. W. Cox. 1992. Management of excess water on duplex soils. Australian Journal of Experimental Agriculture 32: 857-864.
Musgrave, A., M. B. Jackson, AND E. Ling. 1972. Callitriche stem elongation is controlled by ethylene and gibberellin. Nature New Biology 238: 93-96.
Pereira, J. S., AND T. T. Kozlowski. 1977. Variations among woody angiosperms in response to flooding. Physiologia Plantarum 41: 184-192.
Ponnamperuma, F. N. 1972. The chemistry of submerged soil. Advances in Agronomy 24: 29-95.
Prudhomme C., D. Jakob, AND C. Svensson. 2002. Impact of climate change on flooding in the UK: a methodology for estimating uncertainty. 4th International Friend Conference 2002 – Regional hydrology: bridging the gap between research and practice., Cape Town, South Africa, Publication number 274: 109-116.
Quimio, C. A., L. B. Torrizo, T. L. Setter, M. Ellis, A. Grover, E. M. Abrigo, N. P. Oliva, E. S. Ella, A. L. Carpena, O. Ito, W. J. Peacock, E. Dennis, AND S. K. Datta. 2000. Enhancement of submergence tolerance in transgenic rice overproducing pyruvate decarboxylase. Journal of Plant Physiology 156: 516-521.
Saglio, P., M. C. Drew, AND A. Pradet. 1988. Metabolic adaptation to anoxia induced by low (2-4 kPa partial pressure) oxygen pretreatment (hypoxia) in root tips of Zea mays. Plant Physiology 86: 61-66.
Setter, T., AND R. Belford. 1990. Waterlogging: How it reduces plant growth and how plants can overcome its effects. Journal of Agriculture-Western Australia 31: 51-55.
Setter, T. L., AND I. Waters. 2003. Review of prospects for germplasm improvement for waterlogging tolerance in wheat, barley and oats. Plant and Soil 253: 1-34.
STOIMENOVA M., HÄNSCH, R., MENDEL, R., GIMMLER, H. AND KAISER, W.M. 2003. The role of nitrate reduction in the anoxic metabolism of roots I. Characterization of root morphology and normoxic metabolism of wild type tobacco and a transformant lacking root nitrate reductase. Plant and Soil, 253 : 145-153.
Trought, M. C. T., AND M. C. Drew. 1981. Alleviation of injury to young wheat plants in anaerobic solution cultures in relation to the supply of nitrate and other inorganic nutrients. Journal of Experimental Botany 32: 509-522.
Van Der Ploeg, R. R., G. Machulla, D. Hermsmeyer, J. Ilsemann, M. Gieska, AND J. Bachmann. 2002. Changes in land use and the growing number of flash floods in Germany., Agricultural effects on ground and surface waters: research at the edge of science and society. International Association for Horticultural Research publication no. 273, 317-321. International Society for Horticultural Science.
Van Toai, T. T., AND C. S. Bolles. 1991. Postanoxic injury in soybean (Glycine max) seedlings. Plant Physiology 97: 588-592.
Vartapetian, B. B., AND M. B. Jackson. 1997. Plant adaptations to anaerobic stress. Annals of Botany 79: 3-20.
Vartapetian, B. B., P. Mazliak, AND C. Lance. 1978. Lipid biosynthesis in rice coleoptiles grown in the presence or the absence of oxygen. Plant Science Letters 13: 321-328.
Walker, G. E. 1991. Chemical, physical and biological-control of carrot seedling diseases. Plant and Soil 136: 31-39.
Waters, I., W. Armstrong, C. J. Thompson, T. L. Setter, S. Adkins, J. Gibbs, AND H. Greenway. 1989. Diurnal changes in radial oxygen loss and ethanol metabolism in roots of submerged and non-submerged rice seedlings. New Phytologist 113: 439-451.
Webb, T., AND W. Armstrong. 1983. The effects of anoxia and carbohydrates on the growth and viability of rice, pea and pumpkin roots. Journal of Experimental Botany 34: 579-603.
Xia, J. H., P. Saglio, AND J. Roberts. 1995. Nucleotide Levels Do Not Critically Determine Survival of Maize Root Tips Acclimated to a Low-Oxygen Environment. Plant Physiology 108: 589-595.
Yanar, Y., P. E. Lipps, AND I. W. Deep. 1997. Effect of soil saturation, duration and water content on root rot of maize caused by Pythium arrhenomanes. Plant Disease 81: 475-480.