The Mitigation of Heat Stress
Dr. Anthony E. Hall, Botany and Plant Sciences Department University of California, Riverside
Mitigation of stress by crop management
Management methods at sowing
In subtropical zones, cool-season annuals such as lettuce may be sown in the late summer to produce a crop during the fall. The soil can be so hot during the late summer that it reduces the maximum germination that is achieved. Germination of lettuce seed can be inhibited by temperatures of 250 to 330C occurring during a short period of 7 to 12 hours after the seed has begun to imbibe water. The incomplete emergence problem can be overcome by sowing the lettuce seed into dry beds during the day and then sprinkle irrigating the beds during the late afternoon. Sprinkling cools the soil in the seed zone by evaporation, and the seeds imbibe water during the cool conditions of the evening and night enabling most of them to germinate. Another potential solution to this problem is “seed priming” which involves placing the seed in an osmotic solution for several days at moderate temperatures and then drying them. During the priming the seed goes through the initial temperature-sensitive stages of germination with the osmoticum reducing water uptake and preventing radical emergence. Primed seed also has some disadvantages in that it often has a shorter shelf life and is more expensive than normal seed.
In tropical zones, inadequate plant emergence and establishment can limit the productivity of several warm-season annual crops. The soil surface can become very hot. For crops with small seed that are sown shallow, such as sorghum and pearl millet, seed zone temperatures can exceed 450C in some cases and substantially reduce emergence independently of drought effects. Hot soils retard hypocotyl elongation of cowpea and this can have a detrimental effect on emergence, which is aggravated by deep sowing of seeds. Consequently, when soils are hot, seed of cowpea must be sown at a depth that is neither too deep and thus constrain hypocotyl emergence nor too shallow and be too close to the very hot surface.
Choice of sowing date
In temperate or subtropical climatic zones, which have seasonal variations in temperature, sowing date can be varied to increase the probability that annual crop species will escape stressfully high temperatures during subsequent sensitive stages of development. For example, sowing dates can be chosen so that reproductive stages that are particularly sensitive to heat do not occur during periods when stressfully hot weather is most likely to occur. In some subtropical zones the weather can be chilling in early spring and become progressively warmer reaching very hot conditions in the middle of the summer. In these zones warm-season annuals, such as cotton, cowpea and maize that are sown earlier in the spring tend to flower earlier and have a higher probability of escaping hot summer weather during heat-sensitive stages of reproductive development. The earliest dates that sowing should be done depends on the extent of chilling tolerance during germination and emergence of the species and cultivar. Genotypic differences in chilling tolerance during emergence have been detected in cowpea. The chilling tolerance was associated with a dominant effect due to the presence of a specific dehydrin protein in the seed and an independent and additive effective associated with slow electrolyte leakage from seed under chilling conditions (Ismail et al. 1997, 1999). Our subsequent research demonstrated that it is possible to combine chilling tolerance during emergence with heat tolerance during reproductive development in cowpea using conventional hybridization.
Cultivars, irrigation and other management methods
Perennial crop species and cultivars should be chosen that are adapted to the high temperatures likely to occur in the specific location. For both perennial and annual crop species, a degree of escape of high leaf temperatures can be achieved by insuring that maximum transpiration rates are maintained since evaporative cooling can result in leaf temperatures being up to about 80C cooler for rapidly transpiring plants compared with slowly transpiring plants. Plants transpire at maximum rates if their root zones have high levels of soil water and adequate aeration.
High temperature and intense direct solar radiation can cause damage to fruit (e.g. citrus or tomato) and reduce their marketing quality. This can be avoided if fruit is shaded by foliage. Extent of fruit shading by leaves can be manipulated by the choice of cultivars, irrigation methods and fertilizer management methods, and plant training and pruning procedures. Damage to tree trunk cambium by high temperatures can be avoided by spraying the bark of exposed trunks and branches with a reflective white coating.
The Mitigation of Heat Stress by Plant Resistance
The Nature of Resistance to Heat
Genetic resistance to heat is defined as where a genotype is more productive than another genotype in environments where heat stress occurs. This should be distinguished from heat tolerance, which is defined as the relative performance of a plant or plant process under heat compared with performance under optimal temperature. Resistance to heat is more relevant to the needs of farmers than heat tolerance, whereas heat tolerance often is of interest to scientists studying mechanisms of adaptation.
Fischer and Maurer (1978) partitioned stress effects on yield (Y) into parameters measuring sensitivity to stress (S) and the extent of the stress (D) and yield potential (Yp).
Y = Yp (1 – S * D)
Where D = (1 – X/Xp) and X and Xp are the mean yields of all cultivars under stressed and optimal conditions, respectively. Algebraic manipulation shows that:
S = (1 – Y/Yp) / D = (Yp – Y) / (Yp * D)
Since D is constant for a particular trial, S is a measure of the yield decrease due to the stress relative to the potential yield with a low value of S being desirable. Thus S is the inverse of heat tolerance.
The problem with using S as a measure of adaptation to the stress is that there are cases where S has been positively correlated with Yp in that cultivars whose yield was affected little by the stress also had very low yield potential. This means that the cultivars with low S also may have had low stress resistance (Y) and would not be useful for farmers. The correlation between S and Yp also indicates that it may not be possible or easy to combine the desirable features associated with a low S and high yield potential. However, there may be cases where the desirable features associated with low S can be combined with high yield potential. I will provide two examples to show where low S in a genotype may or may not be useful for breeding.
Many landraces of cereals and grain legumes are competitive and have substantial leaf area and photosynthetic source capability but produce relatively few seed in all environments. An example of this is the guineense sorghums that may be found in West Africa. These landraces have low yield potential but when subjected to a late stress due to heat (or drought) that reduces leaf photosynthesis and accelerates leaf senescence they still fill the few grains that had been set. Thus they exhibit little reduction in yield and a low value of S. Breeding to increase the ratio of their reproductive sink to their photosynthetic source could increase the yield potential of the landraces (Yp). But their sensitivity to stress (S) also probably would increase such that little gain may be achieved in their heat resistance (Y).
In contrast, consider cases where heat stress mainly damages reproductive development and particular genotypes tolerate this stress. The stress-tolerant genotypes would have a low S value that may be independent of traits conferring yield potential, such that combining both sets of traits can increase heat resistance. This has been the case with breeding heat-resistant cowpea cultivars using reproductive-stage heat tolerance that is described in the next section. Consequently, the heat sensitivity index S and various heat-tolerance traits must be used with caution, especially for cases where genotypic values for S are positively correlated with yield potential and S depends on traits that influence yield potential.
Greater heat tolerance is defined as being where a specific plant process is damaged less by high tissue temperature and can involve constitutive effects or require acclimation. Tolerance to high soil temperatures during seed germination would appear to require constitutive genetic effects; although the mother-plant environment during seed development and maturation also can influence the heat tolerance of seed during germination. Tolerance to high tissue temperatures during plant emergence and early seedling growth involves both constitutive and acclimation effects. Seedlings subjected to moderately high temperatures synthesize a novel set of proteins that have been called heat-shock proteins, and the plants become more tolerant, in terms of plant survival, to more extreme temperatures (Vierling 1991). These proteins are thought to enable cells to survive the harmful effects of heat by two general types of mechanisms: as molecular chaperones, and by targeting proteins for degradation. As an example of chaperone activity, it has been shown that a specific small heat-shock protein cooperates with other heat-shock proteins to reactivate a heat-denatured protein (Lee and Vierling 2000). Heat-shock proteins do not appear to be the only mechanism whereby plants differ in heat tolerance. For example, genotypes of cowpea have been bred that have substantial differences in heat tolerance during reproductive development but they produced the same set of heat-shock proteins in their leaves when subjected to moderately high temperatures.
For crops that produce fruit and/or seed, including cereals and grain legumes, it is useful to examine whether high temperatures damage the photosynthetic source more than the reproductive sink. In essence we are asking which of these processes is more limiting under hot conditions because enhancing the heat tolerance of this process could increase resistance to heat. Recall that a heat-resistant cultivar is defined as one that has higher productivity than other cultivars when grown in environments where heat stress occurs.
Photosynthetic sources and reproductive sinks, however, may not always be independent factors in adaptation. For spring wheat growing in hot irrigated environments, cultivar differences in grain yield have been positively associated with photosynthetic carbon dioxide fixation rate (Reynolds et al. 1994). Even stronger positive associations were observed between grain yield and stomatal conductance suggesting that more open stomata may be responsible for the higher photosynthetic rates through facilitating the diffusion of carbon dioxide into leaves and reducing leaf temperature bringing it closer to the optimum for photosynthesis. Also, cultivar differences in grain yield of spring wheat growing in a hot irrigated environment have been positively correlated with kernel number per spike (Shpiler and Blum 1991). Processes that determine kernel number per spike may be linked to photosynthesis. Fischer (1985) established that wheat cultivar variation in kernels per m2 was positively correlated with spike dry weight at anthesis and the ratio of solar radiation to temperature for the 30-day period prior to anthesis. Consequently, heat stress effects on photosynthesis can reduce both the photosynthetic source and the magnitude of the reproductive sink making it difficult to determine overall effects on the ratio of the photosynthetic source to the reproductive sink.
Also, photosynthetic capacity and stomatal behavior may be influenced by the extent of the reproductive sink for photosynthate through complex long-term feedback effects. For example, Pima cotton cultivars with greater boll yields under hot irrigated conditions also have higher stomatal conductance and greater carbon dioxide assimilation rates (Cornish et al. 1991, Lu et al. 1994, 1998). Plants that have higher photosynthetic capacity often have higher maximal stomatal conductance and the mechanism for this long-term regulation is unknown (Hall 2001). Explanations for the mechanisms whereby the Pima cottons are heat resistant are complex. The heat-resistant cotton cultivars were bred by selecting for ability to set more bolls on lower nodes under hot, irrigated conditions and not for stomatal or photosynthetic properties (reviewed by Hall 1992). Possible causes for the higher photosynthetic rates of the heat-resistant cotton cultivars include the following. More open stomata enhance the diffusion of carbon dioxide into the leaves. Cooler leaves operate closer to the optimum for photosynthesis. Slower senescence of leaves could enhance photosynthesis. Positive feedback effects on stomata or components of leaf photosynthesis may occur due to the stronger sink strength that results from the increased fruiting.
The sensitivity of photosynthesis and photosystem II to heat may be due to detrimental effects of high temperature on chloroplast membranes. Murukami et al. (2000) developed transgenic tobacco plants with altered chloroplast membranes by silencing the gene encoding chloroplast omega-3 fatty acid desaturase. The transgenic plants had less trienoic fatty acids and more dienoic fatty acids in their chloroplasts than the wild type. The transgenic plants also had greater photosynthesis and grew better than wild-type plants in hot but highly artificial environments. The studies are preliminary in that rigorous tests would include evaluating responses of the transgenic and wild type plants in more natural hot environments and determining the whole-plant mechanisms of any effects on photosynthesis and growth. Highly artificial environments can result in artifacts that do not occur in nature.
Clones of Irish potato have been bred with differences in resistance to heat, in terms of tuber yield (reviewed by Hall 1992). Under hot conditions several processes are inhibited that influence tuber production: the rate of photosynthesis, induction to tuberize and tuberization. Controlled-environment studies demonstrated that these processes are influenced differently by root and shoot temperatures (Reynolds and Ewing 1989). High soil temperature inhibited tuber development and growth under either hot or more optimal shoot temperatures. In contrast, high shoot temperatures caused leaf rolling and accelerated leaf senescence and reduced the induction to tuberize under either hot or more optimal root-zone temperatures. This example, illustrates the importance of considering both root-zone and shoot-zone temperatures when developing techniques for screening for heat tolerance and developing management methods for hot environments.
When plants growing in pots are subjected to high air temperatures both the shoot and the roots are subjected to hot conditions. In contrast, when plants growing in the field are subjected to high air temperatures, the shoot is subjected to more extreme temperatures than the root system. In field conditions, temperature of the soil below about 10 cm is buffered, and does not heat up as much or cool down as much as the air. Consequently, using plants in pots when studying effects of heat stress, can subject roots to unnaturally high temperatures and generate artifacts.
Management practices can influence soil temperatures. For example, compare the effects of frequent sprinkler irrigation and frequent drip irrigation on Irish potato grown on beds in hot environments. Overhead sprinkling will cool the beds more than will drip irrigation, and cooler beds may enhance tuber development and growth while effects on plant water status may be similar for the two systems of management.
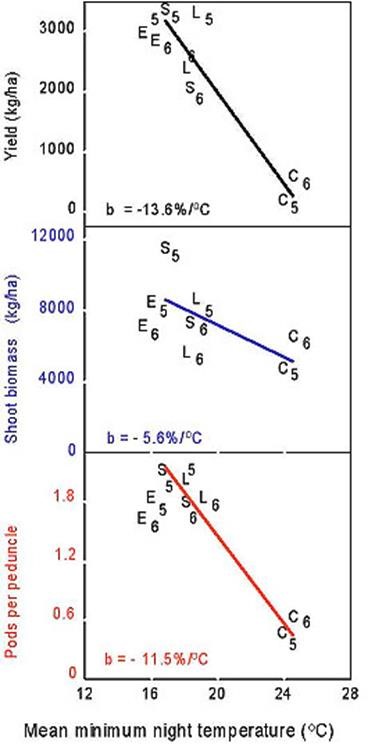
For cowpea, high temperatures have greater detrimental effects on reproductive development and grain yield than they do on biomass production (Fig.3) and presumably photosynthesis. Consequently, breeding to enhance heat tolerance during reproductive development could enhance heat resistance. Cowpea genotypes have been discovered that differ in heat tolerance during reproductive development (Ehlers and Hall 1996) and genetic studies have elucidated the inheritance of this complex trait. Heat-tolerance during early floral bud development and ability to produce flowers was shown to be consistent with the effect of a single recessive gene and have very high heritability (Hall 1993). In contrast, heat-tolerance during pod set in cowpea was shown to be consistent with the effect of a single dominant gene but with strong environmental effects and low narrow-sense and realized heritabilities of 0.26 (Marfo and Hall 1992). For some other species, such as tomato, high temperatures may influence several aspects of reproduction involving both the anther and the stigma and the inheritance of heat tolerance for fruit set probably is more complex than it is for cowpea (reviewed by Hall 1992).
Embryo abortion also is a complex character that is influenced by many stresses, and plant pod-load and age. The discovery that two cowpea genotypes do not exhibit reductions in number of seeds per pod under high night temperatures, even with a substantial pod load (Ehlers and Hall 1998), indicates there may be an opportunity for genetic and breeding studies of heat tolerance during embryo development. Genetic studies demonstrated that heat-induced seed coat browning is consistent with the effect of a single dominant gene that is not linked to the gene conferring heat tolerance during floral bud development (Patel and Hall 1988).
Heat tolerance during reproductive development of cowpea is consistent with the presence of a set of genes that operate in the following developmental sequence. They determine the number of floral buds that develop and produce flowers, the number of these flowers that produce pods, the number of embryos that develop and produce seed, and the quality of the seed that are produced.
Several reproductive processes are particularly sensitive to high night temperatures but some may be sensitive to or aggravated by high day temperatures. In addition, for cowpea, there were greater effects of heat stress on both flower production and pod set under long days compared with short days. This indicates that heat stress may be more damaging to cowpea in subtropical than tropical zones and this has been confirmed in both glasshouse studies (Ehlers and Hall 1998) and field studies.
In some cases, plant breeding can be used to counteract the detrimental heat-induced acceleration of reproductive development. For indeterminate crops, such as most grain legume cultivars, cotton and tomato, the length of the reproductive period can be changed by modifying plant habit and the progression of production of vegetative nodes, branches and reproductive nodes. For example, once reproductive buds have been initiated, a few cowpea genotypes have the ability to produce more vegetative nodes on the main stem alternating with reproductive nodes (Ehlers and Hall 1996). This trait slows down overall reproductive development and may be advantageous in tropical environments with high night temperature.
For determinate crops, such as rice, sorghum and wheat, the progression of vegetative and reproductive structures is not very plastic and the rapid reproductive development caused by high night temperatures substantially reduces their grain yield potential. Where the overall reproductive stage is short, the opportunity for the fixation of photosynthate and its translocation to developing grain also is short. Enhanced translocation to grain of stored photosynthate fixed prior to anthesis may provide a heat-tolerance mechanism for determinate crops that are subjected to heat stress during grain filling. Wheat often experiences heat and drought stress during grain filling when grown in hotter wheat production zones such as may be found in India and the United States. These stresses can substantially reduce the photosynthetic source during grain filling by both accelerating leaf senescence and causing damage to photosynthesis. Enhanced mobilization of stem reserves may be an effective mechanism of tolerance to late season stresses such as heat and drought, especially for determinate crops.
Methods of Breeding for Resistance to Heat
The traditional method for breeding for heat resistance is to grow advanced lines in a hot target production environment and select those lines that have greater yields than current cultivars (this provides a direct measure of heat resistance). This approach is more effective with crop species that can be efficiently yield-tested in small plots, such as wheat, than crops such as cowpea that require larger plots and are more difficult to harvest. This direct approach also is more effective in environments where heat is the only major stress. The presence of other stresses makes the evaluation of heat resistance very difficult. For example, insect pests such as lygus bugs and flower thrips can cause damage to developing flower buds of cowpea that appears similar to the damage caused by high night temperatures. Some slow progress may have been made in enhancing the heat resistance of cowpea in West Africa, however, by breeding programs that selected based upon grain yield in hot target production environments (Ehlers and Hall 1998).
A more efficient approach has been developed for breeding for heat resistance that involves early generation selection for specific traits that confer heat tolerance during reproductive development. The first step in this approach is to discover accessions that have heat-tolerance traits. When searching for useful accessions, a wide range of materials should be evaluated including those that evolved or were developed in cool as well as hot environments. Two cowpea accessions were discovered to have heat tolerance during reproductive development that came from a hot tropical zone in Africa (Warrag and Hall 1983). However, a cowpea breeding line developed for the very cool conditions of Minnesota (MN 13), which was not exposed to heat during its selection, also has heat tolerance during reproductive development. This unexpected finding may be due to associations that are present between reproductive-stage heat tolerance and extreme earliness (Ehlers and Hall 1996). We have speculated that this association may be due to heat susceptibility being caused by the presence of certain phytochromes that suppress or slow down floral development in hot conditions (Ahmed et al. 1993b). The association between extreme earliness and heat tolerance during reproductive development has been observed in other species. Some early tomato cultivars developed for cool environments in Canada and Russia have heat tolerance during fruit set, and some chilling-tolerant snap beans also have heat tolerance (reviewed by Hall 1992).
For some crop species, methods have been developed based on selection for heat-tolerance traits in extremely hot environments that are more effective than selection solely based on yield in hot target production environments. An example of breeding for resistance to heat in cowpea is described that has been shown to be very effective. Emphasis was placed on incorporating heat tolerance during reproductive development. Very hot field and glasshouse environments were used for screening for reproductive-stage heat tolerance. The hot field nursery was achieved by sowing a set of cowpea genotypes that have similar earliness (they initiate floral buds at the same time) in the Coachella Valley of California during the hot season. Often sowing was done about the 20th of June and resulted in an environment where the plants experienced minimum and maximum 24-hour air temperatures of 230 to 270C and 420 to 500C, respectively, for the three-week period beginning one week prior to the start of flowering. In this environment, heat-tolerant day-neutral genotypes begin flowering about 32 days after sowing. The plants also experience long days (14.5 hours) and sunny skies, and are subjected to optimal irrigation, fertilizer application and pest management practices. Plants are selected that produce many early flowers, and have high pod set producing about four pods per peduncle on the first five reproductive nodes on the main stem, well-filled pods and adequate grain quality. In most years many plants can be effectively screened using this field nursery.
Most parts of the world do not have field nurseries with the consistently high night temperatures but otherwise optimal conditions experienced in Coachella Valley in the early summer. Consequently, we also have developed a glasshouse environment for screening cowpea for reproductive-stage heat tolerance. The plants are subjected to minimum and maximum 24-hour air temperatures of 270 and 360C, respectively, and sunny long-day conditions. This glasshouse environment is extremely effective for screening for reproductive-stage heat tolerance but only a few hundred plants can be grown in it compared with the thousands of plants that can be screened in our large field nurseries. The key to the success of the glasshouse nursery is the use of high night temperature rather than high day temperature. One advantage of using a hot glasshouse compared with most hot field conditions is that air temperatures are relatively stable from day to day and over weeks. Consequently, genotypes that begin flowering at different times can be screened reliably. In contrast the summer Coachella Valley environment only is effective for screening genotypes that begin flowering at about the same time because temperatures vary substantially from day to day and exhibit seasonal changes.
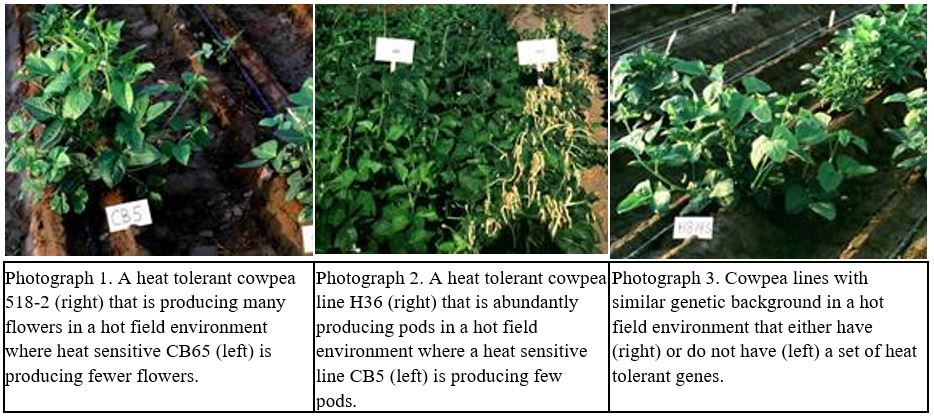
Diverse sets of several hundred early flowering day-neutral cowpea accessions have been screened and three were detected with ability to produce flowers and set pods under hot conditions in the field. The heat-tolerant accessions have many undesirable agronomic traits so it was necessary to cross them with commercial cultivars. We have shown that heat tolerance during early flower development can be incorporated and virtually fixed by a single selection for ability to produce flowers in the F2 or a subsequent generation, consistent with it being conferred by a single recessive gene with high heritability. During the F2 generation, we select plants that produce many flowers and have high pod set and adequate numbers of seeds per pod and seed size and no heat-induced seed coat browning (Photograph 1).
We only can directly screen for heat tolerance under long days in the summer so we have used the fall and winter seasons to advance generations once or twice in an optimal-temperature glasshouse using single-seed or single-plant decent. During the summer we then screen the F4 or F5 lines for heat tolerance. Most of the lines that were selected during the F2 generation have heat tolerance during early flower development in subsequent generations and produce many flowers. In the advanced generations, we emphasize selecting lines and then single plants with uniformly high pod set, adequate numbers of seeds per pod and seed size, and no seed coat browning (Photograph 2).
At least two cycles of family selection are needed to incorporate heat tolerance during pod set, which is consistent with it having dominant gene inheritance and a low heritability.
Six pairs of lines were developed (for example Photographs 2 and 3) that either have or do not have a set of heat-tolerance genes in similar genetic backgrounds. These pairs of lines were evaluated in eight field environments with average night temperatures ranging from cool to very hot but otherwise similar near optimal conditions (Ismail and Hall 1998). The heat-susceptible lines, which included a commercial cultivar, exhibited a 13.5 % decrease in grain yield per 0C increase in average minimum night temperature above 16.50C for the three-week period starting one week prior to first flowering (Fig.3). The heat-tolerant lines had similar grain yields under cooler night temperatures but 50 % greater grain yield and numbers of pods per peduncle than the heat-susceptible lines with average minimum night temperatures of 210C (Fig.4).
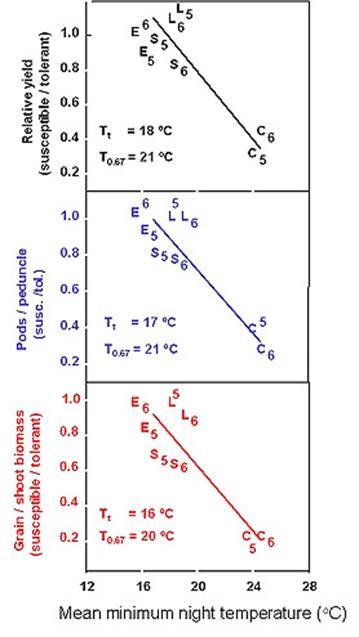
Minimum night temperatures greater than 210C occur in several commercial production zones (Nielsen and Hall 1985a). Advanced lines are then evaluated in multi-location trials conducted in commercial fields and experiment stations in the target production environment. Lines are selected that have consistently high yield, adequate grain quality and other agronomic traits such as resistance to lodging. One of the heat-tolerant lines from the study of Ismail and Hall (1998) that performed well in the multi-location trials now has been released as the cowpea cultivar “California Blackeye 27″ (Ehlers et al. 2000). It should be noted that heat tolerance by itself will not justify release of a new cultivar, the cultivar must have greater grain yield than current cultivars when grown in the target production environment (i.e. greater heat resistance is needed). Farmers often only will accept a new cultivar that has been shown to substantially enhance yields or profits. “California Blackeye 27″ has greater grain yields when conditions are hot at flowering and it also has greater grain yields in some fields due to it having greater resistance to specific pests and diseases than the older cultivars (Ehlers et al. 2000).
When breeding to incorporate heat tolerance or any other trait it is important to evaluate potential negative effects of the trait. In hot and also more moderate temperature environments, the reproductive-stage heat-tolerance genes cause cowpea to be more compact and dwarfed due to their internodes being shorter. At a minimum night temperature of 180C, the heat-susceptible cowpea lines had 50 % longer main stems, and at 220C they had 50 % more vegetative biomass than the heat-tolerant lines (Fig.5 and Photographs 1, 2 and 3).
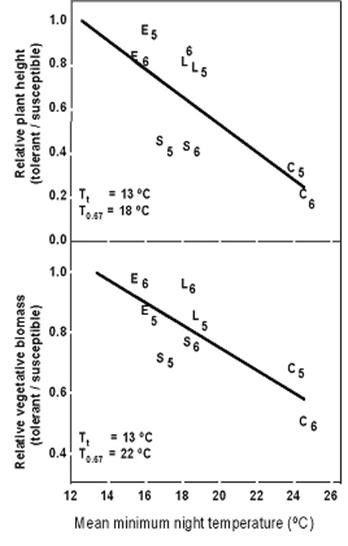
Heat-tolerant semidwarf cowpea lines were compared with standard-height cowpea cultivars under different row spacing (Ismail and Hall 2000). Heat-tolerant semidwarf cowpea lines were less effective than standard-height cultivars at the wide row spacing of 102 cm used by some farmers, more effective with the widely used 76 cm row spacing, and even more effective with a narrow row spacing of 51 cm than standard-height cultivars. Natural selection likely would not favor this type of heat tolerance in that plants with the compact plant habit are not very competitive. In tomato, cultivars with heat-tolerance during reproductive development may tend to be more compact and exhibit less coverage of fruit by leaves, which can enhance damage to the fruit surface and internal tissues caused by excessive solar radiation and temperature. The compactness of the heat-resistant cowpea and tomato cultivars may be due to their greater and earlier partitioning of carbohydrate to fruits, which thereby restricts their vegetative growth compared with heat-susceptible cultivars. The heat-tolerance gene that enhances pod set in cowpea appears to have major effects on plant development.
Screening for the extent of flowering and fruit set in hot conditions can be effective with several crop species, including several grain legumes, tomato and cotton, but some breeders do not have suitable very hot field environments, and hot glasshouse screening can be expensive. Consequently, scientists have tried to develop more efficient indirect screening procedures.
Considerable research effort has been devoted to using slow electrolyte leakage from leaf disks that have been subject to high temperatures as an indication of cell membrane thermostability (MT, or CMS) and heat tolerance (reviewed by Blum 1988 and Hall 1992).
The yield-forming processes that are linked with MT have not been clearly identified. However, for spring and winter wheat, MT was associated with heat tolerance during grain filling (Shanahan et al. 1990, Saadala et al. 1990a, Reynolds et al. 1994) and it may -be possible to obtain useful data from seedling screens (Saadala et al. 1990b). Seedling screens can be very effective because many plants can be screened and selected plants can be crossed.
Positive associations between MT and grain yield under heat stress have been reported for two spring wheat populations (Blum et al. 2001). One population consisted of 98 F8 random recombinant inbred lines (RILs) between a heat-resistant cultivar, Danbata, and a heat-sensitive cultivar, Nacozari. Grain yield was measured for plants growing in the summer at Bet Dagan in Israel. This environment imposed a substantial heat stress and grain yield was only 53 % of that of the same plants growing in the winter at Bet Dagan. The MT was measured on leaves from the same RILs growing in a growth chamber at optimal temperatures and then heat hardened for one day prior to measuring electrolyte leakage from leaf segments. The grain yield of Nacozari was about 100 g m-2 whereas that of Danbata was much higher at 250 g m-2. Grain yields of the RILs varied from about 50 to 300 g m-2. The MT of Nacozari was 31 % whereas that of Danbata was 72 % and the values for the RILs ranged from 22 to 76 %. There was a fairly high and significant positive correlation across the RILs between biomass (r=0.60 p<0.01) or grain yield (r=0.53 p<0.01) measured in the summer and MT. No such correlation was seen for winter production of biomass or grain. The other population consisted of 49 spring wheat F7 breeding lines from 10 different crosses randomly selected from the wheat breeding program at Bet Dagan. Similar methods were used to evaluate these lines as was used with the RILs. Grain yield of the 49 breeding lines ranged from 267 to 370 g m-2 in the hot summer environment. The MT measured with growth chamber plants ranged from 66 to 99 %. There was a significant positive correlation (r=0.56 p<0.01) across lines between MT and grain yield under heat stress. The authors pointed out that the associations were strong but not perfect and that MT should not be used as the exclusive selection criterion. They suggested MT might be a valuable supplemental criterion in final breeding stages or as a rough selection tool in early stages of breeding programs to reduce a large population into the most likely heat-resistant core.
There has been some work on MT for enhancing heat tolerance of other crops (Blum 1988, Hall 1992). For cowpea, electrolyte leakage of leaf disks was negatively associated with reproductive-stage heat tolerance (Ismail and Hall 1999). Subsequent genetic selection experiments by Thiaw and Hall (2004) confirmed that leaf electrolyte leakage under heat stress was negatively correlated with heat tolerance for pod set in cowpea. The leaf-electrolyte-leakage (LEL) protocol that he used consisted of subjecting leaf discs to 46oC for 6 hours in aerated water and then measuring electrical conductivity of the solution followed by boiling the leaf discs and then measuring the electrical conductivity of the solution again. The percentage leakage during heat stress was calculated from the two measurements. Blum (1988) and others have proposed that plants should be heat-hardened prior to sampling tissue, and four measurements of electrolyte leakage are used in calculating MT. Heat-hardening does not appear to be necessary for cowpea in that Thiaw and Hall (2004) and Ismail and Hall (1999) observed useful genotypic differences in LEL with plants grown in a range of different environments. An advantage of the LEL method used by Thiaw (2003) over the MT method used by Blum et al. (2001) is that samples for the LEL method can be taken from plants growing in any field nursery or glasshouse, without the need for acclimating plants. Also, only two measurements of electrolyte leakage are needed with the LEL method so that more plants can be evaluated than with the MT method, which requires four measurements.
Thiaw and hall (2004) selected four populations from the same cross between heat-resistant and heat-susceptible parents that have similar genetic background: those with slow LEL and those with fast LEL, and those with high pod set in hot conditions and those with low pod set in hot conditions. The association between pod set and LEL was strong in that lines selected for slow LEL had high pod set, and lines selected for high pod set had slow LEL. The realized heritability when using slow LEL to indirectly select for heat tolerance during pod set was significantly greater than zero but small, similar to the realized heritability for direct selection for pod set of 0.26 observed by Marfo and Hall (1992). The LEL protocol we used has an advantage over direct selection in that it can be conducted in the off season with plants grown in moderate temperatures. We now propose an improved method for breeding heat-resistant cowpeas. This method consists of direct selection for abundant flowering and pod set in very hot summer field nurseries or glasshouses, followed by indirect selection using slow LEL in the fall and winter with plants grown under moderate temperatures in greenhouses.
Heat tolerance in spring wheat and Pima cotton has been associated with greater stomatal conductance, which can be rapidly detected in plots by a low canopy temperature compared with air temperature using an infrared thermometer (Reynolds et al 1994, Lu et al. 1994 and 1998). However, key tests have not yet been reported for any species that demonstrate whether selecting in segregating populations based on canopy temperature differences confers some heat resistance. Since measurement of canopy temperature differences requires plots of similar genotypes it could only be practiced in relatively advanced generations. This is unfortunate because much progress can be made if selection can be effectively initiated in the first segregating generation using single plants. Also the genotypic differences in canopy temperature that have been reported are relatively small in relation to the errors encountered in these data. This approach probably may not be effective with grain legumes that exhibit diurnal leaf movements because this can increase errors due to the sensor detecting the far infrared radiation emitted from the soil surface. In addition, there are theoretical limits to the extent that stomatal conductance can be increased by selection and enhance crop performance.
For crops where the limiting effect of heat stress involves damage to photosynthesis there is some merit in trying measurements of chlorophyll fluorescence as an indicator of damage to photosystem II. Equipment is available that permits rapid field measurement of the Fv/Fm parameter which provides an estimate of the damage to photosystem II. For this approach, also, key tests have not yet been reported for any species that demonstrate whether selection based on chlorophyll fluorescence is effective in enhancing heat resistance. It should be noted that when determining whether a selection method is effective it also is necessary to determine the efficiency of the method: the costs of the selection procedure in relation to the gains that are made compared with other selection procedures.
In extreme conditions, heat-resistance may depend upon the ability of plants to survive hot environments. The maximum emergence of sorghum and pearl millet seedlings can be substantially reduced by hot soil conditions in tropical Africa and India. Studying heat tolerance during emergence is difficult under field conditions due to the extreme variability in microclimates that is encountered at the soil surface and interactions with variations in soil water status. A laboratory technique has been developed for screening seedling emergence at different controlled soil temperatures which uses infra-red lamps to simulate the heating effects of sunlight and can subject emerging plumules of sorghum and pearl millet to high soil temperatures but without water stress (Soman and Peacock 1985).
In plant breeding it is necessary to take a long term view and consider the environmental and socio-economic conditions likely to be present in future years (Hall and Ziska 2000). Climatic conditions are changing, such as the progressive increases in atmospheric carbon dioxide concentration that are occurring everywhere and will tend to make photosynthesis of C3 plants more effective. Plant photosynthetic systems may require modifications through plant breeding so that they can take full advantage of the elevated atmospheric [CO2]. Also, maintaining a balance between carbohydrate sources and sinks could require selecting plants with greater reproductive sinks (Hall and Ziska 2000). Breeding to maintain a balance will be particularly important for environments and species where stresses, such as high temperatures, cause greater damage to the reproductive sink than the photosynthetic source. The genes for reproductive-stage heat tolerance in cowpea enhance sink strength and harvest index (Ismail and Hall 1998, 2000). Studies in controlled environments indicate these heat-tolerance genes may also enhance responsiveness to elevated atmospheric [CO2] under moderate as well as high night temperatures (Ahmed et al. 1993a).
Advances in biotechnology may make possible some new approaches for breeding for heat resistance. Apomixis could provide resistance to stresses, such as heat, that damage reproductive development, since the seed are produced from maternal tissues and do not require meiosis or fertilization. A type of apomixis would be needed that does not require fertilization of polar nuclei for endosperm development. Through genetic engineering it may be possible to insert the cassette of genes needed to confer facultative apomixis (Jefferson 1993). Facultative apomixis also would provide many other benefits to plant breeding and farmers. These benefits include making hybrid cultivars with seeds that breed true to type. This would make possible the use of hybrid cultivars in many species where currently it is not economically feasible due to the current high cost of producing hybrid seed in relation to the amount of seed that is needed per unit area sown. An additional benefit to farmers would be that they could use their own hybrid seed for several years, which would reduce their seed costs and be of particular benefit for poor farmers.
The literature contains relatively little information on breeding for heat resistance. Some commercial companies have been active in this area but have not published their results. This is unfortunate since their experience with breeding for heat resistance could guide future breeders. Many advanced institutions are located in temperate zones where resistance to cold is more important than resistance to heat. Also, the limited research on heat tolerance conducted in these advanced institutions often has emphasized heat-shock proteins but has not yet led to any methods for breeding for resistance to heat that is based on this information. Breeding for resistance to heat deserves a higher priority than it has been given in the past.
References
Ahmed, F. E. and A. E. Hall. 1993. Heat injury during early floral bud development in cowpea. Crop Sci. 33: 764-767.
Ahmed, F. E., A. E. Hall and D. A. DeMason. 1992. Heat injury during floral development in cowpea (VIGNA UNGUICULATA, FABACEAE). Amer. J. Bot. 79:784-791.
Ahmed, F. E., A. E. Hall and M. A. Madore. 1993a. Interactive effects of high temperature and elevated carbon dioxide concentration on cowpea (Vigna unguiculata (L.) Walp.). Plant, Cell Environ. 16: 835-842.
Ahmed F. E., R. G. Mutters and A. E. Hall. 1993b. Interactive effects of high temperature and light quality on floral bud development in cowpea. Austral. J. Plant Physiol. 20: 661-667.
Al-Khatib, K. and G. M. Paulsen. 1999. High-temperature effects on photosynthetic processes in temperate and tropical cereals. Crop Sci. 39: 119-125.
Blum, A. 1988. Plant Breeding for Stress Environments. CRC Press, Inc., Boca Raton, Florida, pp 223.
Blum, A., N. Klueva and H. T. Nguyen. 2001. Wheat cellular thermotolerance is related to yield under heat stress. Euphytica 117: 117-123.
Cornish, K., J. W. Radin, E. L. Turcotte, Z. Lu and E. Zeiger. 1991. Enhanced photosynthesis and stomatal conductance of pima cotton (Gossypium barbadense L.) bred for increased yield. Plant Physiol. 97: 484-489.
Ehlers, J. D. and A. E. Hall. 1996. Genotypic classification of cowpea based on responses to heat and photoperiod. Crop Sci. 36: 673-679.
Ehlers, J. D. and A. E. Hall. 1998. Heat tolerance of contrasting cowpea lines in short and long days. Field Crops Res. 55: 11-21.
Ehlers, J. D., A. E. Hall, P. N. Patel, P. A. Roberts and W.C. Matthews. 2000. Registration of ‘California Blackeye 27′ cowpea. Crop Sci. 40: 854-855.
Fischer, R. A. 1985. Number of kernels in wheat crops and the influence of solar radiation and temperature. J. Agric. Sci. Camb. 105: 447-461.
Fischer, R. A. and R. Maurer. 1978. Drought resistance in spring wheat cultivars. I. Grain yield responses. Austr. J. Agric. Res. 29: 897-912.
Hall, A. E. 1992. Breeding for heat tolerance. Plant Breed. Rev. 10: 129-168.
Hall, A. E. 1993. Physiology and breeding for heat tolerance in cowpea, and comparison with other crops. Pp. 271-284, in C. G. Kuo (ed.) Adaptation of Food Crops to Temperature and Water Stress, Publ. No. 93-410, Asian Vegetable Research and Development Center, Shanhua, Taiwan.
Hall, A. E. 2001. Crop Responses to Environment. CRC Press LLC, Boca Raton, Florida.
Hall, A. E. and L. H. Ziska. 2000. Crop breeding strategies for the 21st century. Pp. 407-423 in K. R. Reddy and H. F. Hodges (eds.) Climate Change and Global Crop Productivity, CABI Publishing, New York, USA.
Hall, A. E., B. B. Singh and J. D. Ehlers. 1997. Cowpea breeding. Plant Breed. Rev. 15: 215-274.
Ismail, A. M. and A. E. Hall. 1998. Positive and negative effects of heat-tolerance genes in cowpea. Crop Sci. 38: 381-390.
Ismail, A. M. and A. E. Hall. 1999. Reproductive-stage heat tolerance, leaf membrane thermostability and plant morphology in cowpea. Crop Sci. 39: 1762-1768.
Ismail, A. M. and A. E. Hall. 2000. Semidwarf and standard-height cowpea responses to row spacing in different environments. Crop Sci. 40: 1618-1623.
Ismail, A. M., A. E. Hall and T. J. Close. 1997. Chilling tolerance during emergence of cowpea associated with a dehydrin and slow electrolyte leakage. Crop Sci. 37: 1270-1277.
Ismail, A. M., A. E. Hall and T. J. Close. 1999. Allelic variation of a dehydrin gene cosegregates with chilling tolerance during seedling emergence. PNAS 96: 13566-13570.
Jefferson, R. A. 1993. Beyond model systems – new strategies, methods and mechanisms for agricultural research. Ann. New York Acad. Sci. 700: 53-73.
Lee, G. J. and E. Vierling. 2000. A small heat shock protein cooperates with heat shock protein 70 systems to reactivate a heat-denatured protein. Plant Physiol. 122: 189-197.
Lu, Z., R. G. Pearcy, C. O. Qualset and E. Zeiger. 1998. Stomatal conductance predicts yields in irrigated Pima cotton and bread wheat grown at high temperatures. J. Exp. Bot. 49: 453-460.
Lu, Z., J. W. Radin, E. L. Turcotte, R. Percy and E. Zeiger. 1994. High yields in advanced lines of Pima cotton are associated with higher stomatal conductance, reduced leaf area and lower leaf temperature. Physiol. Plant. 92: 266-272.
Marfo, K. O. and A. E. Hall. 1992. Inheritance of heat tolerance during pod set in cowpea. Crop Sci. 32: 912-918.
Murakami, Y., Tsuyama, M., Kobayashi, Y., Kodama, H. and K. Iba. 2000. Trienoic fatty acids and plant tolerance of high temperature. Science 287: 476-479.
Mutters, R. G. and A. E. Hall. 1992. Reproductive responses of cowpea to high temperature during different night periods. Crop Sci. 32: 202-206.
Mutters, R. G., L. G. R. Ferreira and A. E. Hall. 1989a. Proline content of the anthers and pollen of heat-tolerant and heat-sensitive cowpea subjected to different temperatures. Crop Sci. 29: 1497-1500.
Mutters, R. G., A. E. Hall and P. N. Patel. 1989b. Photoperiod and light quality effects on cowpea floral development at high temperatures. Crop Sci. 29: 1501-1505.
Nielsen, C. L. and A. E. Hall. 1985a. Responses of cowpea (Vigna unguiculata [L.] Walp.) in the field to high night temperature during flowering. I. Thermal regimes of production regions and field experimental system. Field Crops Res. 10: 167-179.
Nielsen, C. L. and A. E. Hall. 1985b. Responses of cowpea (Vigna unguiculata [L.] Walp.) in the field to high night temperatures during flowering. II. Plant responses. Field Crop Res.10: 181-196.
Patel, P. N. and A. E. Hall. 1988. Inheritance of heat-induced brown discoloration in seed coats of cowpea. Crop Sci. 28: 929-932.
Reynolds, M. P. and E. E. Ewing. 1989. Effects of high air and soil temperature stress on growth and tuberization in Solanum tuberosum. Annals Bot. 64: 241-247.
Reynolds, M. P., M. Balota, M. I. B. Delgado, I. Amani and R. A. Fischer. 1994. Physiological and morphological traits associated with spring wheat yield under hot, irrigated conditions. Austral. J. Plant Physiol. 21: 717-730.
Saadala, M. M., J. S. Quick and J. F. Shanahan. 1990a. Heat tolerance in winter wheat. II. Membrane thermostability and field performance. Crop Sci. 30: 1248-1252.
Saadala, M. M., J. F. Shanahan and J. S. Quick. 1990b. Heat tolerance in winter wheat. I. Hardening and genetic effects on membrane thermostability. Crop Sci. 30: 1243-1247.
Shanahan, J. F., I. B. Edwards, J. S. Quick and J. R. Fenwick. 1990. Membrane thermostability and heat tolerance of spring wheat. Crop Sci. 30: 247-251.
Shpiler, L. and A. Blum. 1991. Heat tolerance for yield and its components in different wheat cultivars. Euphytica 51: 257-263.
Soman, P. and J. M. Peacock. 1985. A laboratory technique to screen seedling emergence of sorghum and pearl millet at high soil temperature. Expl. Agric. 21: 335-341.
Thiaw, S. 2003. Association between slow leaf-electrolyte-leakage under heat stress and heat tolerance during reproductive development in cowpea. Ph.D. Dissertation, University of California, Riverside.
Thiaw, Samba and Anthony E. Hall. 2004. Comparison of selection for either leaf-electrolyte-leakage or pod set in enhancing heat tolerance and grain yield of cowpea. Field Crops Res.86: 239-253.
Vierling, E. 1991. The roles of heat shock proteins in plants. Ann. Rev. Plant Physiology Plant Mol. Biol. 42: 579-620.
Warrag, M. O. A. and A. E. Hall. 1983. Reproductive responses of cowpea to heat stress: genotypic differences in tolerance to heat at flowering. Crop Sci. 23: 1088-1092.
Warrag, M. O. A. and A. E. Hall. 1984a. Reproductive responses of cowpea [Vigna unguiculata (L.) Walp.] to heat stress. I. Responses to soil and day air temperatures. Field Crops Res. 8: 3-16.
Warrag, M. O. A. and A. E. Hall. 1984b. Reproductive responses of cowpea [Vigna unguiculata (L.) Walp.] to heat stress. II. Responses to night air temperatures. Field Crops Res. 8: 17-33.