The Cell Membrane Stability (CMS) Test
A major impact of plant environmental stress is cellular membrane modification, which results in its perturbed function or total dysfunction. The exact structural and functional modification caused by stress is not fully resolved. However, the cellular membrane dysfunction due to stress is well expressed in increased permeability and leakage of ions out, which can be readily measured by the efflux of electrolytes. Hence the estimation of membrane dysfunction under stress by measuring cellular electrolyte leakage from affected leaf tissue into an aqueous medium is finding a growing use as a measure of CMS and as a screen for stress resistance. The method was initially developed by the late C.Y. Sullivan (University of Nebraska) in the late 1960’s for assessing sorghum and maize heat tolerance. Variations of the method were developed also for cold and desiccation (drought) tolerance. High CMS by this assay was found in many reports to be associated across diverse genetic materials with yield under stress. These reports can be listed by searching our Reference Database for Keywords: leakage yield.
The protocol
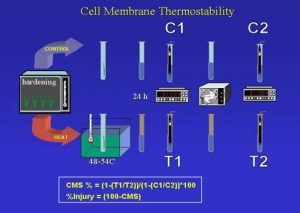
The general protocol involves the application of stress to the leaf after it has been subjected to hardening, followed by the measurement of electrolyte leakage using the conductometric method. The most common application is for heat tolerance and therefore the initial detail is given for heat stress (see schematic outline).
- The plant must be exposed to moderate heat stress for at least 24h before the test in order to allow for hardening (acclimation). The capacity for hardening is a major component of the capacity for tolerance. Hardening can be achieved in the natural field environment, if heat stress occurs, or in the greenhouse or a programmed growth chamber. Exposure of intact plants for 24h to 320C (cool season plants) to 360C (warm season plants) is sufficient, even at low light.
- Leaf discs or pieces of leaf tissue cut with scissors or even whole small leaves are detached and placed in standard glass vials that can accommodate a conductivity electrode (see below). The total area of leaf material per vial is about 15 to 25 cm2. The exact area is not important and it does not have to be the same for all vials. The sample is then washed for 2-3 times with de-ionized water. The water is drained off but samples remain wet so that they would not desiccate. In the case of screening, at least 10 vials (samples) are prepared for each genotype. In that case 5 pairs are taken from five different plants (replicates). For each pair, one vial is designated as treatment (T in Figure) and the other as control (C in Figure).
- The treatment vials are subjected to the heat stress treatment in vitro. (Treatment can also constitute of low temperature in the case of chilling tolerance). They are placed in racks and covered (not stoppered) with ‘Saran’ wrap so as to avoid drying the samples. Racks are placed in thermostated water bath so that the leaf samples will be completely below the water surface level. Temperature is set to a predetermined stress (treatment) temperature and the samples remain in the bath for 1h. The control vials are placed in a rack, covered with Saran wrap and placed at room temperature. The treatment temperature should be such that it will result in average population CMS values around 50%-60% for full separation of the accessions. This temperature will change with the species, the population and the hardening conditions. It is therefore necessary that some representative genotypes will be initially tested for CMS at a range of temperatures (typically: 480-500-520-540C) in order to determine the final treatment temperature.
- After treatment 20cc of deionized water is added to each vial making certain that all leaf materials are submerged. All vials are then placed for incubation at about 100C (typically, on the lowest refrigerator shelf) for 24h. After incubation the samples are equilibrated for 1h at room temperature and the conductivity of the medium is measured by inserting a conductivity electrode into each vial.
- All vials covered with Saran wrap or plastic sheet are placed in an autoclave for 15 min to kill all tissues. Conductivity of all samples is measured after samples are equilibrated to room temperature.
- Calculation: see Figure – where T1 and T2 are treatment conductivities before and after autoclaving and C1 and C2 are the respective control conductivities. Calculated results are often better when each T value is calculated against the average of all C values for the given accession.
CMS for dehydration tolerance
Two methods are used: by applying drought stress to samples either in vivo or in vitro.
In vivo stress: plants of all materials must be stressed to the same relative water content (RWC) of about 60% to 70% (depending on crop species) before being sampled (T1 samples) into stoppered vials and brought to the lab. Samples should not allowed to desiccate between sampling and washing. Control samples are taken from similar leaves of well-watered plants at RWC close to 98%. Once washed, 20 cc of deionized water is added to all samples and they are directly placed for 24h incubation period (#4 above). The remaining procedure is as described above. An example of such work is given by Tripathy et al., 2000. The method requires the monitoring of all entries for leaf RWC when subjected to drought stress, in order to determine time of leaf sampling at the standard RWC.
In vitro stress: This is a simpler procedure. CMS test under drought stress is devised by subjecting the stress samples (T1) to desiccation in vitro in a solution of polyethylene glycol (PEG) (MW 6000 or 8000). Examples are presented by Blum and Ebercon, 1981, Premachandra and Shimada, 1987 and Bajji et al., 2001. This treatment replaces the heat stress treatment as described above and it is performed at room temperature. After 24h (or less) of submergence in PEG the samples are quickly washed to remove PEG from the surface of the samples and then incubated in deionized water as described above. PEG concentration and duration of submergence varies with the experiment, species and PEG. Plants should be subjected to some hardening by drought stress prior to sampling for the stress treatment.
While there is ample evidence to show that heat tolerance in terms of plant production can be reasonably predicted by heat CMS, there is only limited evidence if any that plant production under drought stress is related to CMS under PEG desiccation, partly because this association was not evaluated as extensively as for heat. CMS under PEG stress is not recommended yet as a consensus protocol for drought resistance.








